Abstract
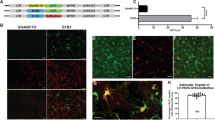
During HIV-1 reverse transcription, central initiation of the plus-strand DNA at the central polypurine tract (cPPT) and central termination at the central termination sequence (CTS) lead to the formation of a three-stranded DNA structure: the HIV-1 central DNA flap. We recently reported that the DNA flap acts as a cis-active determinant of HIV-1 genome nuclear import. Commonly employed HIV-1–derived vectors (HR vectors) lack the central DNA flap. Here we report that the insertion of this DNA flap sequence into HR vectors (TRIP vectors) improves gene transduction in neural cells, ex vivo and in vivo, in rat brain. When neural cells are exposed to increasing concentrations of TRIP vector particles, transgene expression correlates with the dose of vector. This effect contrasts with the plateau observed when using an HR vector. We further demonstrate that the increase of in vivo transduction efficiency obtained with TRIP vectors is due to the stimulation of their genome nuclear import.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gartner, S. et al. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233, 215–219 (1986).
Ho, D., Rota, T. & Hirsch, M. Infection of monocyte/macrophages by human T lymphotropic virus type III. J. Clin. Invest. 77, 1712–1715 (1986).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Kim, V., Mitrophanous, K., Kingsman, S. & Kingsman, A. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J. Virol. 72, 811–816 (1998).
Charneau, P. & Clavel, F. A single-stranded gap in human immunodeficiency virus unintegrated linear DNA defined by a central copy of the polypurine tract. J. Virol. 65, 2415–2421 (1991).
Zennou, V. et al. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101, 173–185 (2000).
Charneau, P., Alizon, M. & Clavel, F. A second origin of plus strand synthesis is required for optimal HIV replication. J. Virol 66, 2814–2820 (1992).
Charneau, P. et al. HIV-1 reverse transcription: a termination step at the center of the genome. J. Mol. Biol. 241, 651–662 (1994).
Follenzi, A., Ailles, L.E., Bakovic, S., Geuna, M. & Naldini, L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 25, 217–222 (2000).
Cisterni, C., Henderson, C.E., Aebischer, P., Pettmann, B. & Deglon, N. Efficient gene transfer and expression of biologically active glial cell line-derived neurotrophic factor in rat motoneurons transduced with lentiviral vectors. J. Neurochem. 74, 1820–1828 (2000).
Zufferey, R., Nagy, D., Mandel, R., Naldini, L. & Trono, D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15, 871–875 (1997).
Yee, J.K. et al. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 91, 9564–9568 (1994).
Barre-Sinoussi, F. et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871 (1983).
Levi, S., Vannier, C. & Triller, A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J. Cell Sci. 111, 335–345 (1998).
Buc-Caron, M.H. Neuroepithelial progenitor cells explanted from human fetal brain proliferate and differentiate in vitro. Neurobiol. Dis. 2, 37–47 (1995).
Ridet, J.L. et al. Toward autologous ex vivo gene therapy for the central nervous system with human adult astrocytes. Hum. Gene Ther. 10, 271–280 (1999).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular cloning: a laboratory manual. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY; 1989).
Acknowledgements
We thank N. Taylor for critical reading of the manuscript, D. De Saint-Jan for the rESE, and F. Lachapelle for his kind assistance. V.Z. was supported by a fellowship from Association Française contre les Myopathies and C.Se. from Vaincre les Maladies Lysosomales. This work was supported by grants from Agence Nationale pour la Recherche contre le SIDA and Association Française contre les Myopathies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zennou, V., Serguera, C., Sarkis, C. et al. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol 19, 446–450 (2001). https://doi.org/10.1038/88115
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/88115
This article is cited by
-
HIV-1 sequences in lentiviral vector genomes can be substantially reduced without compromising transduction efficiency
Scientific Reports (2021)
-
The PINK1 kinase-driven ubiquitin ligase Parkin promotes mitochondrial protein import through the presequence pathway in living cells
Scientific Reports (2019)
-
Human glia can both induce and rescue aspects of disease phenotype in Huntington disease
Nature Communications (2016)
-
Local inactivation of Gpr88 in the nucleus accumbens attenuates behavioral deficits elicited by the neonatal administration of phencyclidine in rats
Molecular Psychiatry (2015)
-
Comparison Between Several Integrase-defective Lentiviral Vectors Reveals Increased Integration of an HIV Vector Bearing a D167H Mutant
Molecular Therapy - Nucleic Acids (2014)