Abstract
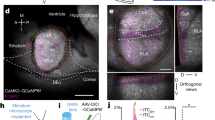
The brain circuits underlying behavioral fear have been extensively studied over the last decades. Although the vast majority of experimental studies assess fear as a transient state of apprehension in response to a discrete threat, such phasic states of fear can shift to a sustained anxious apprehension, particularly in face of diffuse cues with unpredictable environmental contingencies. Unpredictability, in turn, is considered an important variable contributing to anxiety disorders. The networks of the extended amygdala have been suggested keys to the control of phasic and sustained states of fear, although the underlying synaptic pathways and mechanisms remain poorly understood. Here, we show that the endocannabinoid system acting in synaptic circuits of the extended amygdala can explain the fear response profile during exposure to unpredictable threat. Using fear training with predictable or unpredictable cues in mice, combined with local and cell-type-specific deficiency and rescue of cannabinoid type 1 (CB1) receptors, we found that presynaptic CB1 receptors on distinct amygdala projections to bed nucleus of the stria terminalis (BNST) are both necessary and sufficient for the shift from phasic to sustained fear in response to an unpredictable threat. These results thereby identify the causal role of a defined protein in a distinct brain pathway for the temporal development of a sustained state of anxious apprehension during unpredictability of environmental influences, reminiscent of anxiety symptoms in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sylvers P, Lilienfeld SO, LaPrairie JL . Differences between trait fear and trait anxiety: implications for psychopathology. Clin Psychol Rev 2011; 31: 122–137.
Grupe DW, Nitschke JB . Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 2013; 14: 488–501.
Avery SN, Clauss JA, Blackford JU . The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 2015; 41: 126–141.
Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C . Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage 2011; 55: 389–400.
Pape H-C, Paré D . Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 2010; 90: 419–463.
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A . Amygdala inhibitory circuits and the control of fear memory. Neuron 2009; 62: 757–771.
Johansen JP . Neuroscience: anxiety is the sum of its parts. Nature 2013; 496: 174–175.
Duvarci S, Paré D . Amygdala microcircuits controlling learned fear. Neuron 2014; 82: 966–980.
Maren S, Quirk GJ . Neuronal signalling of fear memory. Nat Rev Neurosci 2004; 5: 844–852.
Tovote P, Fadok JP, Lüthi A . Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015; 16: 317–331.
Davis M, Walker DL, Miles L, Grillon C . Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 2010; 35: 105–135.
Grillon C, Baas JP, Lissek S, Smith K, Milstein J . Anxious responses to predictable and unpredictable aversive events. Behav Neurosci 2004; 118: 916–924.
Daldrup T, Remmes J, Lesting J, Gaburro S, Fendt M, Meuth P et al. Expression of freezing and fear-potentiated startle during sustained fear in mice. Genes Brain Behav 2015; 14: 281–291.
Seidenbecher T, Remmes J, Daldrup T, Lesting J, Pape H-C . Distinct state anxiety after predictable and unpredictable fear training in mice. Behav Brain Res 2016; 304: 20–23.
Foa EB, Zinbarg R, Rothbaum BO . Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull 1992; 112: 218–238.
Mineka S, Kihlstrom JF . Unpredictable and uncontrollable events: a new perspective on experimental neurosis. J Abnorm Psychol 1978; 87: 256–271.
Walker DL, Miles LA, Davis M . Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1291–1308.
Davis M, Walker DL, Lee Y . Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci 1997; 352: 1675–1687.
Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 2013; 496: 219–223.
Haufler D, Nagy FZ, Paré D . Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem 2013; 20: 633–641.
Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010; 468: 277–282.
Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A . Switching on and off fear by distinct neuronal circuits. Nature 2008; 454: 600–606.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al. Distinct extended amygdala circuits for divergent motivational states. Nature 2013; 496: 224–228.
Daldrup T, Lesting J, Meuth P, Seidenbecher T, Pape H-C . Neuronal correlates of sustained fear in the anterolateral part of the bed nucleus of stria terminalis. Neurobiol Learn Mem 2016; 131: 137–146.
Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci 2004; 24: 9953–9961.
Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y . Endocannabinoid signaling and synaptic function. Neuron 2012; 76: 70–81.
Kamprath K, Romo-Parra H, Häring M, Gaburro S, Doengi M, Lutz B et al. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 2011; 36: 652–663.
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002; 418: 530–534.
Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L et al. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci 2011; 14: 1542–1547.
Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K et al. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron 2014; 81: 1111–1125.
Riebe CJ, Wotjak CT . Endocannabinoids and stress. Stress 2011; 14: 384–397.
Morena M, Patel S, Bains JS, Hill MN . Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 2015; 41: 80–102.
Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS . Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci 2010; 30: 14980–14986.
Lutz B, Marsicano G, Maldonado R, Hillard CJ . The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 2015; 16: 705–718.
Guggenhuber S, Monory K, Lutz B, Klugmann M . AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One 2010; 5: e15707.
Jüngling K, Lange MD, Szkudlarek HJ, Lesting J, Erdmann FS, Doengi M et al. Increased GABAergic efficacy of central amygdala projections to neuropeptide S neurons in the brainstem during fear memory retrieval. Neuropsychopharmacology 2015; 40: 2753–2763.
Lange MD, Jüngling K, Paulukat L, Vieler M, Gaburro S, Sosulina L et al. Glutamic acid decarboxylase 65: a link between GABAergic synaptic plasticity in the lateral amygdala and conditioned fear generalization. Neuropsychopharmacology 2014; 39: 2211–2220.
Puente N, Elezgarai I, Lafourcade M, Reguero L, Marsicano G, Georges F et al. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS One 2010; 5: e8869.
Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wörtge S et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci 2013; 33: 10264–10277.
Walker DL, Davis M . Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 2008; 213: 29–42.
Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003; 302: 84–88.
Miles L, Davis M, Walker D . Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology 2011; 36: 1563–1574.
Walker DL, Toufexis DJ, Davis M . Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 2003; 463: 199–216.
Alger BE . Not too excited? Thank your endocannabinoids. Neuron 2006; 51: 393–395.
Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005; 123: 493–505.
Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci 2014; 17: 407–415.
Higa KK, Ji B, Buell MR, Risbrough VB, Powell SB, Young JW et al. Restoration of Sp4 in forebrain GABAergic neurons rescues hypersensitivity to ketamine in Sp4 hypomorphic mice. Int J Neuropsychopharmacol 2015; 18: pyv063.
Hammack SE, Mania I, Rainnie DG . Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol 2007; 98: 638–656.
Hazra R, Guo J-D, Ryan SJ, Jasnow AM, Dabrowska J, Rainnie DG . A transcriptomic analysis of type I-III neurons in the bed nucleus of the stria terminalis. Mol Cell Neurosci 2011; 46: 699–709.
Rodríguez-Sierra OE, Turesson HK, Paré D . Contrasting distribution of physiological cell types in different regions of the bed nucleus of the stria terminalis. J Neurophysiol 2013; 110: 2037–2049.
Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y et al. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci 2012; 32: 18035–18046.
Chevaleyre V, Castillo PE . Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 2003; 38: 461–472.
Herrmann MJ, Boehme S, Becker MPI, Tupak S V, Guhn A, Schmidt B et al. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum Brain Mapp 2015; 37: 1091–1102.
Straube T, Mentzel H-J, Miltner WHR . Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage 2007; 37: 1427–1436.
Acknowledgements
The authors thank Julia Schröer, Elke Naß, Birgit Herrenpoth, and Svetlana Kiesling, Anisa Kosan and Andrea Conrad for excellent technical assistance, and Annika Lüttjohann, Michael Döngi and Peter Blässe for helpful advice. The project was funded by the German research foundation (CRC-TRR58, TPA03 to H.C.P, and TPA04 to B.L. and H.C.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Lange, M., Daldrup, T., Remmers, F. et al. Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol Psychiatry 22, 1422–1430 (2017). https://doi.org/10.1038/mp.2016.156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.156
This article is cited by
-
Increased cannabis intake during the COVID-19 pandemic is associated with worsening of depression symptoms in people with PTSD
BMC Psychiatry (2022)
-
Cannabinoid Type 1 Receptors in the Basolateral Amygdala Regulate ACPA-Induced Place Preference and Anxiolytic-Like Behaviors
Neurochemical Research (2022)
-
Functional deletion of neuropeptide Y receptors type 2 in local synaptic networks of anteroventral BNST facilitates recall and increases return of fear
Molecular Psychiatry (2021)
-
Lateral hypothalamus involvement in control of stress response by bed nucleus of the stria terminalis endocannabinoid neurotransmission in male rats
Scientific Reports (2021)
-
Endocannabinoid and dopaminergic system: the pas de deux underlying human motivation and behaviors
Journal of Neural Transmission (2021)