Abstract
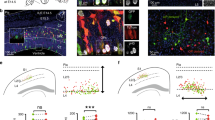
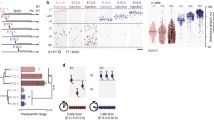
Neurons in the mammalian neocortex are organized into functional columns1,2. Within a column, highly specific synaptic connections are formed to ensure that similar physiological properties are shared by neuron ensembles spanning from the pia to the white matter. Recent studies indicate that synaptic connectivity in the neocortex is sparse and highly specific3,4,5,6,7,8 to allow even adjacent neurons to convey information independently9,10,11,12. How this fine-scale microcircuit is constructed to create a functional columnar architecture at the level of individual neurons largely remains a mystery. Here we investigate whether radial clones of excitatory neurons arising from the same mother cell in the developing neocortex serve as a substrate for the formation of this highly specific microcircuit. We labelled ontogenetic radial clones of excitatory neurons in the mouse neocortex by in utero intraventricular injection of enhanced green fluorescent protein (EGFP)-expressing retroviruses around the onset of the peak phase of neocortical neurogenesis. Multiple-electrode whole-cell recordings were performed to probe synapse formation among these EGFP-labelled sister excitatory neurons in radial clones and the adjacent non-siblings during postnatal stages. We found that radially aligned sister excitatory neurons have a propensity for developing unidirectional chemical synapses with each other rather than with neighbouring non-siblings. Moreover, these synaptic connections display the same interlaminar directional preference as those observed in the mature neocortex. These results indicate that specific microcircuits develop preferentially within ontogenetic radial clones of excitatory neurons in the developing neocortex and contribute to the emergence of functional columnar microarchitectures in the mature neocortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hubel, D. H. & Wiesel, T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. (Lond.) 160, 106–154 (1962)
Mountcastle, V. B., Davies, P. W. & Berman, A. L. Response properties of neurons of cat’s somatic sensory cortex to peripheral stimuli. J. Neurophysiol. 20, 374–407 (1957)
Kozloski, J., Hamzei-Sichani, F. & Yuste, R. Stereotyped position of local synaptic targets in neocortex. Science 293, 868–872 (2001)
Markram, H., Lubke, J., Frotscher, M., Roth, A. & Sakmann, B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. (Lond.) 500, 409–440 (1997)
Song, S., Sjostrom, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005)
Thomson, A. M., West, D. C., Wang, Y. & Bannister, A. P. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro . Cereb. Cortex 12, 936–953 (2002)
Yoshimura, Y., Dantzker, J. L. & Callaway, E. M. Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873 (2005)
Yoshimura, Y. & Callaway, E. M. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nature Neurosci. 8, 1552–1559 (2005)
Ohki, K. et al. Highly ordered arrangement of single neurons in orientation pinwheels. Nature 442, 925–928 (2006)
Ohki, K., Chung, S., Ch'ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005)
Sato, T. R., Gray, N. W., Mainen, Z. F. & Svoboda, K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol. 5, e189 (2007)
Maldonado, P. E., Godecke, I., Gray, C. M. & Bonhoeffer, T. Orientation selectivity in pinwheel centers in cat striate cortex. Science 276, 1551–1555 (1997)
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001)
Noctor, S. C., Martinez-Cerdeno, V., Ivic, L. & Kriegstein, A. R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nature Neurosci. 7, 136–144 (2004)
Miyata, T., Kawaguchi, A., Okano, H. & Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727–741 (2001)
Malatesta, P., Hartfuss, E. & Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263 (2000)
Tamamaki, N., Nakamura, K., Okamoto, K. & Kaneko, T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci. Res. 41, 51–60 (2001)
Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–83 (1972)
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988)
Kornack, D. R. & Rakic, P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron 15, 311–321 (1995)
Cepko, C. L. et al. Studies of cortical development using retrovirus vectors. Cold Spring Harb. Symp. Quant. Biol. 55, 265–278 (1990)
Walsh, C. & Cepko, C. L. Clonally related cortical cells show several migration patterns. Science 241, 1342–1345 (1988)
Walsh, C. & Cepko, C. L. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature 362, 632–635 (1993)
Hensch, T. K. Critical period plasticity in local cortical circuits. Nature Rev. Neurosci. 6, 877–888 (2005)
Micheva, K. D. & Beaulieu, C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J. Comp. Neurol. 373, 340–354 (1996)
Stern, E. A., Maravall, M. & Svoboda, K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo . Neuron 31, 305–315 (2001)
Sjostrom, P. J., Turrigiano, G. G. & Nelson, S. B. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164 (2001)
Thomson, A. M. & Bannister, A. P. Interlaminar connections in the neocortex. Cereb. Cortex 13, 5–14 (2003)
Douglas, R. J. & Martin, K. A. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 27, 419–451 (2004)
Kriegstein, A., Noctor, S. & Martinez-Cerdeno, V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nature Rev. Neurosci. 7, 883–890 (2006)
Acknowledgements
We thank A. L. Joyner, J. Kaltschmidt, Y. Hayashi and Y. Chin for comments on the manuscript; and S. C. Noctor and F. H. Gage for providing the 293gp NIT–GFP retrovirus packaging cell line; and the Shi laboratory members for insightful discussion. We are grateful for support from the March of Dimes Foundation, the Whitehall Foundation, the Klingenstein Foundation, the DANA Foundation, the Autism Speaks Foundation, the National Alliance for Research on Schizophrenia and Depression (NARSAD) and the National Institutes of Health (to S.-H.S.).
Author Contributions Y.-C.Y. and S.-H.S. conceived the experiments. Y.-C.Y. conducted the electrophysiology and imaging experiments. R.S.B and X.W. helped with in utero virus injection. Y.-C.Y. and S.-H.S. analysed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-5 with Legends (PDF 1812 kb)
Rights and permissions
About this article
Cite this article
Yu, YC., Bultje, R., Wang, X. et al. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504 (2009). https://doi.org/10.1038/nature07722
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07722
This article is cited by
-
Nanomaterial-based microelectrode arrays for in vitro bidirectional brain–computer interfaces: a review
Microsystems & Nanoengineering (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
-
Patterned cPCDH expression regulates the fine organization of the neocortex
Nature (2022)
-
A protein pattern regulates the positions and connections of neuronal cells
Nature (2022)
-
DotMotif: an open-source tool for connectome subgraph isomorphism search and graph queries
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.