Abstract
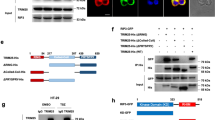
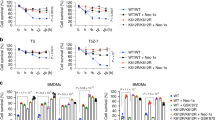
Tumour-necrosis factor (TNF) receptor-associated factor 2 (TRAF2) is a key component in NF-κB signalling triggered by TNF-α1,2. Genetic evidence indicates that TRAF2 is necessary for the polyubiquitination of receptor interacting protein 1 (RIP1)3 that then serves as a platform for recruitment and stimulation of IκB kinase, leading to activation of the transcription factor NF-κB. Although TRAF2 is a RING domain ubiquitin ligase, direct evidence that TRAF2 catalyses the ubiquitination of RIP1 is lacking. TRAF2 binds to sphingosine kinase 1 (SphK1)4, one of the isoenzymes that generates the pro-survival lipid mediator sphingosine-1-phosphate (S1P) inside cells. Here we show that SphK1 and the production of S1P is necessary for lysine-63-linked polyubiquitination of RIP1, phosphorylation of IκB kinase and IκBα, and IκBα degradation, leading to NF-κB activation. These responses were mediated by intracellular S1P independently of its cell surface G-protein-coupled receptors. S1P specifically binds to TRAF2 at the amino-terminal RING domain and stimulates its E3 ligase activity. S1P, but not dihydro-S1P, markedly increased recombinant TRAF2-catalysed lysine-63-linked, but not lysine-48-linked, polyubiquitination of RIP1 in vitro in the presence of the ubiquitin conjugating enzymes (E2) UbcH13 or UbcH5a. Our data show that TRAF2 is a novel intracellular target of S1P, and that S1P is the missing cofactor for TRAF2 E3 ubiquitin ligase activity, indicating a new paradigm for the regulation of lysine-63-linked polyubiquitination. These results also highlight the key role of SphK1 and its product S1P in TNF-α signalling and the canonical NF-κB activation pathway important in inflammatory, antiapoptotic and immune processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karin, M. & Gallagher, E. TNFR signaling: ubiquitin-conjugated TRAFfic signals control stop-and-go for MAPK signaling complexes. Immunol. Rev. 228, 225–240 (2009)
Hayden, M. S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008)
Lee, T. H., Shank, J., Cusson, N. & Kelliher, M. A. The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 279, 33185–33191 (2004)
Xia, P. et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-α signaling. J. Biol. Chem. 277, 7996–8003 (2002)
Bhoj, V. G. & Chen, Z. J. Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 (2009)
Paugh, S. W. et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 112, 1382–1391 (2008)
Spiegel, S. & Milstien, S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Rev. Mol. Cell Biol. 4, 397–407 (2003)
Kimura, T. et al. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high-density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 281, 37457–37467 (2006)
Ki, S. H., Choi, M. J., Lee, C. H. & Kim, S. G. Gα12 specifically regulates COX-2 induction by sphingosine 1-phosphate: Role for JNK-dependent ubiquitination and degradation of IκBα. J. Biol. Chem. 282, 1938–1947 (2007)
Pitson, S. M. et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 22, 5491–5500 (2003)
Zhao, Y. et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: Role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 282, 14165–14177 (2007)
Van Brocklyn, J. R. et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled orphan receptor edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 142, 229–240 (1998)
Giussani, P. et al. Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-Golgi trafficking of ceramide. Mol. Cell. Biol. 26, 5055–5069 (2006)
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006)
Wu, C. J., Conze, D. B., Li, T., Srinivasula, S. M. & Ashwell, J. D. Sensing of Lys-63-linked polyubiquitination by NEMO is a key event in NF-κB activation. Nature Cell Biol. 8, 398–406 (2006)
Wang, H. et al. Analysis of nondegradative protein ubiquitylation with a monoclonal antibody specific for lysine-63-linked polyubiquitin. Proc. Natl Acad. Sci. USA 105, 20197–20202 (2008)
Newton, K. et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678 (2008)
Shi, C. S. & Kehrl, J. H. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 278, 15429–15434 (2003)
Habelhah, H. et al. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-κB. EMBO J. 23, 322–332 (2004)
Li, S., Wang, L. & Dorf, M. E. PKC phosphorylation of TRAF2 mediates IKKα/β recruitment and K63-linked polyubiquitination. Mol. Cell 33, 30–42 (2009)
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008)
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008)
Xu, M., Skaug, B., Zeng, W. & Chen, Z. J. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFα and IL-1β. Mol. Cell 36, 302–314 (2009)
Yin, Q., Lamothe, B., Darnay, B. G. & Wu, H. Structural basis for the lack of E2 interaction in the RING domain of TRAF2. Biochemistry 48, 10558–10567 (2009)
Morris, G. M. et al. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 (1998)
Hait, N. C. et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 (2009)
Maceyka, M., Alvarez, S. E., Milstien, S. & Spiegel, S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol. Cell. Biol. 28, 5687–5697 (2008)
Allende, M. L. et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 (2004)
Paugh, B. S. et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCδ, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 22, 455–465 (2008)
Theiss, A. L. et al. Prohibitin inhibits tumor necrosis factor α-induced nuclear factor-κB nuclear translocation via the novel mechanism of decreasing importin α3 expression. Mol. Biol. Cell 20, 4412–4423 (2009)
Acknowledgements
We thank Z. J. Chen, B. Darnay and M. Karin for HA–ubiquitin and TRAF constructs, and R. Proia for the Sphk1−/− mice. This work was supported by grants from the National Institute of Health (R37GM043880, R01CA61774, R01AI50094, U19AI077435 to S.S.) and in part by the Ministry of Scientific and Technology of China (2009CB918502 to C.L.).
Author information
Authors and Affiliations
Contributions
S.E.A. and K.B.H. planned and performed most experiments, with assistance from N.C.H., G.M.S., E.Y.K., J.A. and M.M.; C.L. and H.J. performed molecular docking; T.K. contributed to the planning of the experiments; S.M. and S.S. conceived the study, contributed to planning of the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Tables 1-10 w (PDF 4949 kb)
Rights and permissions
About this article
Cite this article
Alvarez, S., Harikumar, K., Hait, N. et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465, 1084–1088 (2010). https://doi.org/10.1038/nature09128
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature09128
This article is cited by
-
Unraveling the involvement of WRKY TFs in regulating plant disease defense signaling
Planta (2024)
-
The Sphingosine 1-Phosphate Axis: an Emerging Therapeutic Opportunity for Endometriosis
Reproductive Sciences (2023)
-
Cellular inhibitor of apoptosis 2 (cIAP2) restricts neuroinflammation during experimental autoimmune encephalomyelitis
Journal of Neuroinflammation (2022)
-
Computational analysis of 4-1BB-induced NFκB signaling suggests improvements to CAR cell design
Cell Communication and Signaling (2022)
-
TNF-α impairs EP4 signaling through the association of TRAF2-GRK2 in primary fibroblast-like synoviocytes
Acta Pharmacologica Sinica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.