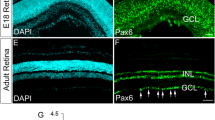
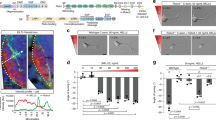
Abstract
Repulsive guidance molecule (RGM) is an axon guidance protein that repels retinal axons upon activation of the neogenin receptor. To understand the functions of RGM–neogenin complexes in vivo, we used gene transfer technology to perturb their expression in the developing neural tube of chick embryos. Surprisingly, neogenin over-expression or RGM down-expression in the neural tube induces apoptosis. Neogenin pro-apoptotic activity in immortalized neuronal cells and in the neural tube is associated with the cleavage of its cytoplasmic domain by caspases. Thus neogenin is a dependence receptor inducing cell death in the absence of RGM, whereas the presence of RGM inhibits this effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Monnier, P.P. et al. RGM is a repulsive guidance molecule for retinal axons. Nature 419, 392–395 (2002).
Hamburger, V. & Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morph. 88, 49–92 (1951).
Vielmetter, J., Kayyem, J.F., Roman, J.M. & Dreyer, W.J. Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation, is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J. Cell Biol. 127, 2009–2020 (1994).
Keino-Masu, K. et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996).
Rajagopalan, S. et al. Neogenin mediates the action of repulsive guidance molecule. Nature Cell Biol. DOI:10.1038/ncb1156 (2004).
Llambi, F., Causeret, F., Bloch-Gallego, E. & Mehlen, P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 20, 2715–2722 (2001).
Thibert, C. et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science 301, 843–846 (2003).
Mehlen, P. et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395, 801–804 (1998).
Mehlen, P. & Bredesen, B.E. The dependence receptor hypothesis. Apoptosis 4, 37–49 (2003).
Katahira, T. & Nakamura, H. Gene silencing in chick embryos with a vector-based small interfering RNA system. Dev. Growth Differ. 45, 361–367 (2003).
Bai, J. et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nature Neurosci. 6, 1277–1283 (2003).
Hong, K. et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 925–941 (1999).
Cho, K.R. & Fearon, E.R. DCC: linking tumor suppressor genes and altered cell surface interactions in cancer? Curr. Opin. Genet. Dev. 5, 72–78 (1995).
Thiebault, K. et al. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc. Natl Acad. Sci. USA 100, 4173–4178 (2003).
Zaidi, A.U. et al. Bcl-X(L)-caspase-9 interactions in the developing nervous system: evidence for multiple death pathways. J. Neurosci. 21, 169–175 (2001).
Srinivasan, K., Strickland, P., Valdes, A., Shin, G.C. & Hinck, L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell 4, 371–382 (2003).
Gad, J.M., Keeling, S.L., Wilks, A.F., Tan, S.S. & Cooper, H.M. The expression patterns of guidance receptors, DCC and Neogenin, are spatially and temporally distinct throughout mouse embryogenesis. Dev. Biol. 192, 258–273 (1997).
Niederkofler, V., Salie, R., Sigrist, M. & Arber, S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J. Neurosci. 24, 808–818 (2004).
Matsunaga, E., Araki, I. & Nakamura, H. Pax6 defines the di-mesencephalic boundary by repressing En1 and Pax2. Development 127, 2357–2365 (2000).
Hughes, S.H., Greenhouse, J.J., Petropoulos, C.J. & Sutrave, P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 61, 3004–3012 (1987).
Fekete, D.M. & Cepko, C.L. Retroviral infection coupled with tissue transplantation limits gene transfer in the chicken embryo. Proc. Natl Acad. Sci. USA 90, 2350–2354 (1993).
Funahashi, J.-i. et al. Role of Pax-5 in the regulation of a mid-hindbrain organizer's activity. Dev. Growth Differ. 41, 59–72 (1999).
Bally-Cuif, L., Wassef, M. Ectopic induction and reorganization of Wnt-1 expression in quail/chick chimeras. Development 120, 3379–3394 (1994).
Marillat, V. et al. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J. Comp. Neurol. 442, 130–155 (2002).
Acknowledgements
We thank E. Fearon, S. H. Hughes and G. S. Salvesen for reagents, and H. Nakamura for expression vectors and discussions. We also thank C. Guix for technical assistance. A.C. is supported by Retina France, the Schlumberger foundation and Association pour la Recherche sur le Cancer (ARC). P.M. is supported by the Ligue Contre le Cancer, the Schlumberger foundation and the National Institute of Health (NIH). This work is also supported by a grant from the N.I.H. to S.M.S and by a BioChance grant from the Bundesministerium für Bildung und Forschung to Migragen. S.T.D. is supported by a post-doctoral fellowship from the Schlumberger foundation, E.M. is a recipient of HFSP Long-Term Fellowship. S.M.S. is an investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Matsunaga, E., Tauszig-Delamasure, S., Monnier, P. et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol 6, 749–755 (2004). https://doi.org/10.1038/ncb1157
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1157
This article is cited by
-
Inhibition of the Nogo-pathway in experimental spinal cord injury: a meta-analysis of 76 experimental treatments
Scientific Reports (2023)
-
Decreased DNA Methylation of RGMA is Associated with Intracranial Hypertension After Severe Traumatic Brain Injury: An Exploratory Epigenome-Wide Association Study
Neurocritical Care (2022)
-
An antibody to RGMa promotes regeneration of cochlear synapses after noise exposure
Scientific Reports (2021)
-
Repulsive guidance molecule acts in axon branching in Caenorhabditis elegans
Scientific Reports (2021)
-
RGMa can induce skeletal muscle cell hyperplasia via association with neogenin signalling pathway
In Vitro Cellular & Developmental Biology - Animal (2021)