Abstract
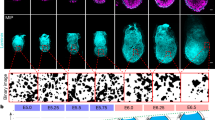
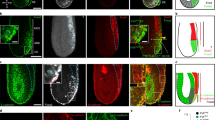
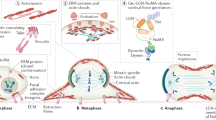
Molecular and cellular mechanisms of epithelial–mesenchymal transition (EMT), crucial in development and pathogenesis, are still poorly understood. Here we provide evidence that distinct cellular steps of EMT occur sequentially during gastrulation. Basement membrane (BM) breakdown is the first recognizable step and is controlled by loss of basally localized RhoA activity and its activator neuroepithelial-transforming-protein-1 (Net1). Failure of RhoA downregulation during EMT leads to BM retention and reduction of its activity in normal epithelium leads to BM breakdown. We also show that this is in part mediated by RhoA-regulated basal microtubule stability. Microtubule disruption causes BM breakdown and its stabilization results in BM retention. We propose that loss of Net1 before EMT reduces basal RhoA activity and destabilizes basal microtubules, causing disruption of epithelial cell–BM interaction and subsequently, breakdown of the BM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Hay, E. D. An overview of epithelio–mesenchymal transformation. Acta Anat. (Basel) 154, 8–20 (1995).
Huber, M. A., Kraut, N. & Beug, H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 (2005).
Thiery, J. P. Epithelial–mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15, 740–746 (2003).
Zavadil, J. & Bottinger, E. P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nature Rev. Mol. Cell Biol. 7, 131–142 (2006).
Hatta, K. & Takeichi, M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320, 447–449 (1986).
Zohn, I. E. et al. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell 125, 957–969 (2006).
Cano, A. et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2, 76–83 (2000).
Lee, J. M., Dedhar, S., Kalluri, R. & Thompson, E. W. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 172, 973–981 (2006).
Jaffe, A. B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Fukata, M. & Kaibuchi, K. Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nature Rev. Mol. Cell Biol. 2, 887–897 (2001).
Van Aelst, L. & Symons, M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 16, 1032–1054 (2002).
Ozdamar, B. et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307, 1603–1609 (2005).
Keller, R. Cell migration during gastrulation. Curr. Opin. Cell Biol. 17, 533–541 (2005).
Stern, C. D. Gastrulation: from cells to embryo (Cold Spring Harbor Laboratory Press New York, 2004).
Tam, P. P. & Behringer, R. R. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 68, 3–25 (1997).
Sheng, G., dos Reis, M. & Stern, C. D. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell 115, 603–613 (2003).
Iimura, T. & Pourquie, O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature 442, 568–571 (2006).
Gustafson, T. & Wolpert, L. Studies on the cellular basis of morphogenesis in the sea urchin embryo. Gastrulation in vegetalized larvae. Exp. Cell Res. 22, 437–449 (1961).
Newgreen, D. F. & Minichiello, J. Control of epitheliomesenchymal transformation. I. Events in the onset of neural crest cell migration are separable and inducible by protein kinase inhibitors. Dev. Biol. 170, 91–101 (1995).
Watanabe, T. et al. Tet-on inducible system combined with in ovo electroporation dissects multiple roles of genes in somitogenesis of chicken embryos. Dev. Biol. 305, 625–636 (2007).
Fuse, T. et al. Conditional activation of RhoA suppresses the epithelial to mesenchymal transition at the primitive streak during mouse gastrulation. Biochem. Biophys. Res. Commun. 318, 665–672 (2004).
Benink, H. A. & Bement, W. M. Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 168, 429–439 (2005).
Chan, A. M., Takai, S., Yamada, K. & Miki, T. Isolation of a novel oncogene, NET1, from neuroepithelioma cells by expression cDNA cloning. Oncogene 12, 1259–1266 (1996).
Alberts, A. S. & Treisman, R. Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J. 17, 4075–4085 (1998).
Miyakoshi, A., Ueno, N. & Kinoshita, N. Rho guanine nucleotide exchange factor xNET1 implicated in gastrulation movements during Xenopus development. Differentiation 72, 48–55 (2004).
Schmidt, A. & Hall, A. The Rho exchange factor Net1 is regulated by nuclear sequestration. J. Biol. Chem. 277, 14581–14588 (2002).
Qin, H. et al. Characterization of the biochemical and transforming properties of the neuroepithelial transforming protein 1. J. Biol. Chem. 280, 7603–7613 (2005).
Aktories, K., Braun, U., Rosener, S., Just, I. & Hall, A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem. Biophys. Res. Commun. 158, 209–213 (1989).
Fukata, Y. et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nature Cell Biol. 4, 583–591 (2002).
Palazzo, A. F., Cook, T. A., Alberts, A. S. & Gundersen, G. G. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nature Cell Biol. 3, 723–729 (2001).
Fukata, M. et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109, 873–85 (2002).
Lansbergen, G. et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5β. Dev. Cell 11, 21–32 (2006).
Mimori-Kiyosue, Y. et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168, 141–153 (2005).
Wen, Y. et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nature Cell Biol. 6, 820–830 (2004).
van Horck, F. P., Ahmadian, M. R., Haeusler, L. C., Moolenaar, W. H. & Kranenburg, O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J. Biol. Chem. 276, 4948–4956 (2001).
Birkenfeld, J. et al. GEF–H1 Modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell 12, 699–712 (2007).
Halfter, W., Dong, S., Yip, Y. P., Willem, M. & Mayer, U. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 22, 6029–6040 (2002).
Stern, C. D., Manning, S. & Gillespie, J. I. Fluid transport across the epiblast of the early chick embryo. J. Embryol. Exp. Morphol. 88, 365–384 (1985).
Gumbiner, B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Rev. Mol. Cell Biol. 6, 622–634 (2005).
Nakaya, Y., Kuroda, S., Katagiri, Y. T., Kaibuchi, K. & Takahashi, Y. Mesenchymal–epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev. Cell 7, 425–438 (2004).
Liu, J. P. & Jessell, T. M. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 125, 5055–5067 (1998).
Liu, A. X., Rane, N., Liu, J. P. & Prendergast, G. C. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell Biol. 21, 6906–6912 (2001).
Hakem, A. et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 (2005).
Bement, W. M., Benink, H. A. & von Dassow, G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol. 170, 91–101 (2005).
Pertz, O., Hodgson, L., Klemke, R. L. & Hahn, K. M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069–1072 (2006).
Simoes, S. et al. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development 133, 4257–4267 (2006).
Wu, K. Y. et al. Local translation of RhoA regulates growth cone collapse. Nature 436, 1020–1024 (2005).
Yonemura, S., Hirao-Minakuchi, K. & Nishimura, Y. Rho localization in cells and tissues. Exp. Cell Res. 295, 300–314 (2004).
Sasai, N., Nakazawa, Y., Haraguchi, T. & Sasai, Y. The neurotrophin-receptor-related protein NRH1 is essential for convergent extension movements. Nature Cell Biol. 6, 741–748 (2004).
Acknowledgements
We thank Fujio Toki for the initial analyses of fibronectin, laminin and 6G7; Yoshiko Takahashi for bringing the phenotype of RhoA cells to our attention; Kathy Joubin for sharing Net1 information; Cantas Alev for generating the Net1 probe; Shigenobu Yonemura and Kazuyo Misaki for help with EM; Masatoshi Takeichi, Yoshiko Takahashi, William Bement, Jeffrey Frost, Alan Hall, Kozo Kaibuchi and Shigenobu Yonemura for sharing reagents; Noriaki Sasai and Yoshiki Sasai for help with Xenopus experiments. Claudio Stern, Donald Newgreen, Kozo Kaibuchi, Masatoshi Takeichi, Fumio Matsuzaki, Shigeo Hayashi and Shigenobu Yonemura for critical comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Y.N. and G.S. designed the experiments and analysed the data; Y.N, E.W.S. and Y.W. performed the experiments; G.S wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5, S6, S7 and S8 (PDF 2967 kb)
Supplementary Information
Supplementary Movie 1 (AVI 2394 kb)
Supplementary Information
Supplementary Movie 2 (AVI 2394 kb)
Rights and permissions
About this article
Cite this article
Nakaya, Y., Sukowati, E., Wu, Y. et al. RhoA and microtubule dynamics control cell–basement membrane interaction in EMT during gastrulation. Nat Cell Biol 10, 765–775 (2008). https://doi.org/10.1038/ncb1739
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1739
This article is cited by
-
Signaling pathways in rheumatoid arthritis: implications for targeted therapy
Signal Transduction and Targeted Therapy (2023)
-
RHOA protein expression correlates with clinical features in gastric cancer: a systematic review and meta-analysis
BMC Cancer (2022)
-
Brain metastatic outgrowth and osimertinib resistance are potentiated by RhoA in EGFR-mutant lung cancer
Nature Communications (2022)
-
How mechanisms of stem cell polarity shape the human cerebral cortex
Nature Reviews Neuroscience (2022)
-
Loss of KAP3 decreases intercellular adhesion and impairs intracellular transport of laminin in signet ring cell carcinoma of the stomach
Scientific Reports (2022)