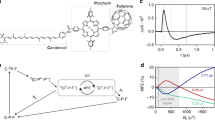
Abstract
Magnetic fields as weak as the Earth's can change the yields of radical pair reactions even though the energies involved are orders of magnitude smaller than the thermal energy, kBT, at room temperature. Proposed as the source of the light-dependent magnetic compass in migratory birds, the radical pair mechanism is thought to operate in cryptochrome flavoproteins in the retina. Here we demonstrate that the primary magnetic field effect on flavin photoreactions can be amplified chemically by slow radical termination reactions under conditions of continuous photoexcitation. The nature and origin of the amplification are revealed by studies of the intermolecular flavin–tryptophan and flavin–ascorbic acid photocycles and the closely related intramolecular flavin–tryptophan radical pair in cryptochrome. Amplification factors of up to 5.6 were observed for magnetic fields weaker than 1 mT. Substantial chemical amplification could have a significant impact on the viability of a cryptochrome-based magnetic compass sensor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wiltschko, R. & Wiltschko, W. Avian magnetic compass: its functional properties and physical basis. Curr. Zool. 56, 265–276 (2010).
Mouritsen, H. in Neurosciences—from Molecule to Behavior: a University Textbook (eds Galizia, C. G. & Lledo, P.-M. ) 427–443 (Springer-Verlag, 2013).
Kishkinev, D. A. & Chernetsov, N. S. Magnetoreception systems in birds: a review of current research. Biol. Bull. Rev. 5, 46–62 (2015).
Ritz, T., Adem, S. & Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718 (2000).
Rodgers, C. T. & Hore, P. J. Chemical magnetoreception in birds: a radical pair mechanism. Proc. Natl Acad. Sci. USA 106, 353–360 (2009).
Mouritsen, H. & Hore, P. J. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr. Opin. Neurobiol. 22, 343–352 (2012).
Chaves, I. et al. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364 (2011).
Giovani, B., Byrdin, M., Ahmad, M. & Brettel, K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nature Struct. Biol. 10, 489–490 (2003).
Zeugner, A. et al. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J. Biol. Chem. 280, 19437–19440 (2005).
Biskup, T. et al. Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angew. Chem. Int. Ed. 48, 404–407 (2009).
Maeda, K. et al. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl Acad. Sci. USA 109, 4774–4779 (2012).
Niessner, C. et al. Magnetoreception: activated cryptochrome 1a concurs with magnetic orientation in birds. J. R. Soc. Interface 10, 20130638 (2013).
Niessner, C., Denzau, S., Peichl, L., Wiltschko, W. & Wiltschko, R. Magnetoreception in birds: I. Immunohistochemical studies concerning the cryptochrome cycle. J. Exp. Biol. 217, 4221–4224 (2014).
Lee, A. A. et al. Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 11, 20131063 (2014).
Hogben, H. J., Efimova, O., Wagner-Rundell, N., Timmel, C. R. & Hore, P. J. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 480, 118–122 (2009).
Solov'yov, I. A. & Schulten, K. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 96, 4804–4813 (2009).
Conrad, K. S., Manahan, C. C. & Crane, B. R. Photochemistry of flavoprotein light sensors. Nature Chem. Biol. 10, 801–809 (2014).
Gao, J. et al. Trp triad-dependent rapid photoreduction is not required for the function of Arabidopsis CRY1. Proc. Natl Acad. Sci. USA 112, 9135–9140 (2015).
Steiner, U. E. & Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 89, 51–147 (1989).
Liedvogel, M. & Mouritsen, H. Cryptochromes—a potential magnetoreceptor: what do we know and what do we want to know? J. R. Soc. Interface 7, S147–S162 (2010).
Dodson, C. A., Hore, P. J. & Wallace, M. I. A radical sense of direction: signalling and mechanism in cryptochrome magnetoreception. Trends Biochem. Sci. 38, 435–446 (2013).
Mouritsen, H. et al. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc. Natl Acad. Sci. USA 101, 14294–14299 (2004).
Möller, A., Sagasser, S., Wiltschko, W. & Schierwater, B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91, 585–588 (2004).
Niessner, C. et al. Avian ultraviolet/violet cones identified as probable magnetoreceptors. PLoS ONE 6, e20091 (2011).
Gegear, R. J., Casselman, A., Waddell, S. & Reppert, S. M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 454, 1014–1018 (2008).
Yoshii, T., Ahmad, M. & Helfrich-Forster, C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 7, 813–819 (2009).
Gegear, R. J., Foley, L. E., Casselman, A. & Reppert, S. M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 463, 804–807 (2010).
Foley, L. E., Gegear, R. J. & Reppert, S. M. Human cryptochrome exhibits light-dependent magnetosensitivity. Nature Commun. 2, 356 (2011).
Fedele, G. et al. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster. PLoS Genet. 10, e1004804 (2014).
Fedele, G., Green, E. W., Rosato, E. & Kyriacou, C. P. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nature Commun. 5, 4391 (2014).
Marley, R., Giachello, C. N. G., Scrutton, N. S., Baines, R. A. & Jones, A. R. Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci. Rep. 4, 5799 (2014).
Ahmad, M., Galland, P., Ritz, T., Wiltschko, R. & Wiltschko, W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 225, 615–624 (2007).
Harris, S.-R. et al. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 6, 1193–1205 (2009).
Henbest, K. B. et al. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl Acad. Sci. USA 105, 14395–14399 (2008).
Binhi, V. N. Stochastic dynamics of magnetosomes and a mechanism of biological orientation in the geomagnetic field. Bioelectromagnetics 27, 58–63 (2006).
Winklhofer, M. & Kirschvink, J. L. A quantitative assessment of torque-transducer models for magnetoreception. J. R. Soc. Interface 7, S273–S289 (2010).
Cai, J. Quantum probe and design for a chemical compass with magnetic nanostructures. Phys. Rev. Lett. 106, 100501 (2011).
Miura, T., Maeda, K. & Arai, T. Effect of coulomb interaction on the dynamics of the radical pair in the system of flavin mononucleotide and hen egg-white lysozyme (HEWL) studied by a magnetic field effect. J. Phys. Chem. B 107, 6474–6478 (2003).
Murakami, M., Maeda, K. & Arai, T. Dynamics of intramolecular electron transfer reaction of FAD studied by magnetic field effects on transient absorption spectra. J. Phys. Chem. A 109, 5793–5800 (2005).
Neil, S. R. T. et al. Broadband cavity-enhanced detection of magnetic field effects in chemical models of a cryptochrome magnetoreceptor. J. Phys. Chem. B 118, 4177–4184 (2014).
Evans, E. W. et al. Sensitive fluorescence-based detection of magnetic field effects in photoreactions of flavins. Phys. Chem. Chem. Phys. 17, 18456–18463 (2015).
Dodson, C. A. et al. Fluorescence-detected magnetic field effects on radical pair reactions from femtolitre volumes. Chem. Commun. 51, 8023–8026 (2015).
Beardmore, J. P., Antill, L. M. & Woodward, J. R. Optical absorption and magnetic field effect based imaging of transient radicals. Angew. Chem. 54, 8494–8497 (2015).
Hore, P. J. & Broadhurst, R. W. Photo-CIDNP of biopolymers. Prog. Nucl. Magn. Reson. Spectrosc. 25, 345–402 (1993).
Morozova, O. B. et al. Time resolved CIDNP study of electron transfer reactions in proteins and model compounds. Mol. Phys. 100, 1187–1195 (2002).
Mok, K. H. & Hore, P. J. Photo-CIDNP NMR methods for studying protein folding. Methods 34, 75–87 (2004).
Atkins, P. W. & Evans, G. T. Magnetic-field effects on chemiluminescent fluid solutions. Mol. Phys. 29, 921–935 (1975).
Mani, T. & Vinogradov, S. A. Magnetic field effects on triplet–triplet annihilation in solutions: modulation of visible/NIR luminescence. J. Phys. Chem. Lett. 4, 2799–2804 (2013).
Hore, P. J. & Kaptein, R. Proton nuclear magnetic-resonance assignments and surface accessibility of tryptophan residues in lysozyme using photochemically induced dynamic nuclear polarization spectroscopy. Biochemistry 22, 1906–1911 (1983).
Vaish, S. P. & Tollin, G. Flash photolysis of flavins. V. Oxidation and disproportionation of flavin radicals. J. Bioenerg. 2, 61–72 (1971).
Massey, V. Activation of molecular-oxygen by flavins and flavoproteins. J. Biol. Chem. 269, 22459–22462 (1994).
Brocklehurst, B. Spin correlation in geminate recombination of radical ions in hydrocarbons. 1. Theory of magnetic-field effect. J. Chem. Soc. Faraday Trans. II 72, 1869–1884 (1976).
Timmel, C. R., Till, U., Brocklehurst, B., McLauchlan, K. A. & Hore, P. J. Effects of weak magnetic fields on free radical recombination reactions. Mol. Phys. 95, 71–89 (1998).
Weber, S. et al. Origin of light-induced spin-correlated radical pairs in cryptochrome. J. Phys. Chem. B 114, 14745–14754 (2010).
Song, S. H. et al. Absorption and fluorescence spectroscopic characterization of cryptochrome 3 from Arabidopsis thaliana. J. Photochem. Photobiol. B 85, 1–16 (2006).
Shirdel, J., Zirak, P., Penzkofer, A., Breitkreuz, H. & Wolf, E. Absorption and fluorescence spectroscopic characterisation of the circadian blue-light photoreceptor cryptochrome from Drosophila melanogaster (dCry). Chem. Phys. 352, 35–47 (2008).
Weaver, J. C., Vaughan, T. E. & Astumian, R. D. Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature 405, 707–709 (2000).
Maeda, K. et al. Following radical pair reactions in solution: a step change in sensitivity using cavity ring-down detection. J. Am. Chem. Soc. 133, 17807–17815 (2011).
Rodgers, C. T., Norman, S. A., Henbest, K. B., Timmel, C. R. & Hore, P. J. Determination of radical re-encounter probability distributions from magnetic field effects on reaction yields. J. Am. Chem. Soc. 129, 6746–6755 (2007).
Hoang, N. et al. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 6, e160 (2008).
Bouly, J. P. et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 282, 9383–9391 (2007).
Acknowledgements
We thank N. Baker for expert technical assistance. E.W.E. is indebted to the Engineering and Physical Sciences Research Council and SABMiller plc for his doctoral scholarship. C.A.D. gratefully acknowledges her current Imperial College Junior Research Fellowship. We are grateful to the following for financial support: the Defense Advanced Research Projects Agency (QuBE: N66001-10-1-4061), the European Research Council (under the European Union's 7th Framework Programme, FP7/2007-2013/ERC grant agreement No. 340451), the Air Force Office of Scientific Research (Air Force Materiel Command, USAF award No. FA9550-14-1-0095) and the EMF Biological Research Trust.
Author information
Authors and Affiliations
Contributions
D.R.K. and E.W.E. contributed equally to this work. D.R.K., E.W.E. and V.D. designed and performed the experiments. D.R.K. and E.W.E. analysed the data. C.A.D. advised on the production of the AtCry1 samples. M.I.W. helped oversee the fluorescence microscopy experiments. C.R.T., S.R.M. and P.J.H. coordinated the study. P.J.H., D.R.K. and E.W.E. wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2505 kb)
Rights and permissions
About this article
Cite this article
Kattnig, D., Evans, E., Déjean, V. et al. Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nature Chem 8, 384–391 (2016). https://doi.org/10.1038/nchem.2447
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2447