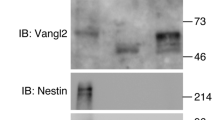
Abstract
Planar cell polarity (PCP) refers to coordinated polarization of cells within the plane of a cell sheet. A conserved signaling pathway is required for the establishment of PCP in epithelial tissues and for polarized cellular rearrangements known as convergent extension. During PCP signaling, core PCP proteins are sorted asymmetrically along the polarization axis; this sorting is thought to direct coordinated downstream morphogenetic changes across the entire tissue. Here, we show that a gene encoding a ciliary protein (a 'ciliary gene'), Ift88, also known as Polaris, is required for establishing epithelial PCP and for convergent extension of the cochlear duct of Mus musculus. We also show that the proper positioning of ciliary basal bodies and the formation of polarized cellular structures are disrupted in mice with mutant ciliary proteins ('ciliary mutants'), whereas core PCP proteins are partitioned normally along the polarization axis. Thus, our data uncover a distinct requirement for ciliary genes in basal body positioning and morphological polarization during PCP regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wallingford, J.B. et al. Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405, 81–85 (2000).
Wang, J. et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133, 1767–1778 (2006).
Wang, Y., Guo, N. & Nathans, J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26, 2147–2156 (2006).
Ybot-Gonzalez, P. et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134, 789–799 (2007).
Montcouquiol, M. et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423, 173–177 (2003).
Curtin, J.A. et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 13, 1129–1133 (2003).
Lu, X. et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430, 93–98 (2004).
Wang, J. et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 37, 980–985 (2005).
Qian, D. et al. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121–133 (2007).
Cotanche, D.A. & Corwin, J.T. Stereociliary bundles reorient during hair cell development and regeneration in the chick cochlea. Hear. Res. 52, 379–402 (1991).
Tilney, L.G., Tilney, M.S. & DeRosier, D.J. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 8, 257–274 (1992).
Denman-Johnson, K. & Forge, A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J. Neurocytol. 28, 821–835 (1999).
Kikuchi, T., Tonosaki, A. & Takasaka, T. Development of apical-surface structures of mouse otic placode. Acta Otolaryngol. (Stockh.) 106, 200–207 (1988).
Sobkowicz, H.M., Slapnick, S.M. & August, B.K. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J. Neurocytol. 24, 633–653 (1995).
Kikuchi, K. & Hilding, D. The development of the organ of Corti in the mouse. Acta Otolaryngol. (Stockh.) 60, 207–222 (1965).
Ross, A.J. et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135–1140 (2005).
Murcia, N.S. et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127, 2347–2355 (2000).
Pazour, G.J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Pazour, G.J. et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J. Cell Biol. 157, 103–113 (2002).
Yoder, B.K. et al. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am. J. Physiol. Renal Physiol. 282, F541–F552 (2002).
Cano, D.A., Murcia, N.S., Pazour, G.J. & Hebrok, M. orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131, 3457–3467 (2004).
Taulman, P.D., Haycraft, C.J., Balkovetz, D.F. & Yoder, B.K. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol. Biol. Cell 12, 589–599 (2001).
Robert, A. et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 120, 628–637 (2007).
Haycraft, C.J. et al. Intraflagellar transport is essential for endochondral bone formation. Development 134, 307–316 (2007).
Hebert, J.M. & McConnell, S.K. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev. Biol. 222, 296–306 (2000).
Pauley, S., Lai, E. & Fritzsch, B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev. Dyn. 235, 2470–2482 (2006).
Badea, T.C., Wang, Y. & Nathans, J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J. Neurosci. 23, 2314–2322 (2003).
Montcouquiol, M., Crenshaw, E.B.III & Kelley, M.W. Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 29, 363–386 (2006).
Seifert, J.R. & Mlodzik, M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126–138 (2007).
Jones, C. & Chen, P. Planar cell polarity signaling in vertebrates. Bioessays 29, 120–132 (2007).
Montcouquiol, M. et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265–5275 (2006).
Deans, M.R. et al. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J. Neurosci. 27, 3139–3147 (2007).
Scholey, J.M. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 19, 423–443 (2003).
Sandoz, D. et al. Organization and functions of cytoskeleton in metazoan ciliated cells. Biol. Cell 63, 183–193 (1988).
Beisson, J. & Jerka-Dziadosz, M. Polarities of the centriolar structure: morphogenetic consequences. Biol. Cell 91, 367–378 (1999).
Hagiwara, H., Kano, A., Aoki, T., Ohwada, N. & Takata, K. Localization of γ-tubulin to the basal foot associated with the basal body extending a cilium. Histochem. J. 32, 669–671 (2000).
Park, T.J., Haigo, S.L. & Wallingford, J.B. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 38, 303–311 (2006).
Kondo, S. et al. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J. Cell Biol. 125, 1095–1107 (1994).
Lin, F. et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc. Natl. Acad. Sci. USA 100, 5286–5291 (2003).
Beech, P.L. et al. Localization of kinesin superfamily proteins to the connecting cilium of fish photoreceptors. J. Cell Sci. 109, 889–897 (1996).
Liu, A., Wang, B. & Niswander, L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111 (2005).
Huangfu, D. & Anderson, K.V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325–11330 (2005).
Schneider, L. et al. PDGFRα signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861–1866 (2005).
Simons, M. et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 37, 537–543 (2005).
Heisenberg, C.P. et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81 (2000).
Boisvieux-Ulrich, E., Laine, M.C. & Sandoz, D. The orientation of ciliary basal bodies in quail oviduct is related to the ciliary beating cycle commencement. Biol. Cell 55, 147–150 (1985).
Mitchell, B., Jacobs, R., Li, J., Chien, S. & Kintner, C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature 447, 97–101 (2007).
Absalon, S. et al. Basal body positioning is controlled by flagellum formation in Trypanosoma brucei. PLoS ONE 2, e437 (2007).
Kibar, Z. et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28, 251–255 (2001).
Acknowledgements
We thank W. Sale for discussions about the project, B. Shur, A. Fritz and T. Caspary for critical comments on the manuscript and S. Mark for technical support. We also thank S. Guadagnini and M.-C. Prévost from the Electron Microscopy Group (PFME) at the Pasteur Institute for their technical advice. Antibody against Fzd3 was a gift from J. Nathans (Johns Hopkins). This project is supported by NIH research grants to P.C. (RO1 DC005213 and DC007423) and B.K.Y. (RO1 DK065655), a postdoctoral fellowship to C.J. (F32DC008731), a Woodruff Foundation grant to P.C., a postdoctoral fellowship to I.F. (INSERM) and grants from Fondation R. & G. Strittmatter and from Ernst-Jung Stiftung für Medizin to C.P.
Author information
Authors and Affiliations
Contributions
C.J. carried out most of the analysis and played a pivotal role in progressively designing the experiments. V.C.R. and B.B. prepared Ift88flox/floxCreER and Kif3aflox/floxCreER samples. I.F. and C.P. contributed electron microscopy data. The study was initiated by B.K.Y and P.C., and carried out under the direction of P.C. C.J. and P.C. wrote the manuscript, which was critically commented upon and revised by B.K.Y. and C.P. D.Q. generated Vangl2GFP animals.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 730 kb)
Supplementary Video 1
Three-dimensional images of cellular microtubules in a wild-type cochlea. Images of the cellular microtubules from three outer hair cells in a wild-type cochlea were acquired using the Zeiss LSM510 confocal microscope, as described in the text (Fig. 6). Serial 0.5μm optical sections were acquired from the apical-most cell surface moving basally through the organ of corti and projected into three dimensions using NIH ImageJ software. This video complements Figure 6 and provides a more complete view of the apical microtubules. (MOV 43 kb)
Supplementary Video 2
Three-dimensional images of cellular microtubules in an IFT88CKO/CKO mutant cochlea. Images of the cellular microtubules from three outer hair cells in an IFT88CKO/CKO mutant cochlea were acquired using the Zeiss LSM510 confocal microscope, as described in the text (Fig. 6). Serial 0.5μm optical sections were acquired from the apical-most cell surface moving basally through the organ of corti and projected into three dimensions using NIH ImageJ software. This video complements Figure 6 and provides a more complete view of the apical microtubules. (MOV 89 kb)
Rights and permissions
About this article
Cite this article
Jones, C., Roper, V., Foucher, I. et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 40, 69–77 (2008). https://doi.org/10.1038/ng.2007.54
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2007.54
This article is cited by
-
Primary cilia as dynamic and diverse signalling hubs in development and disease
Nature Reviews Genetics (2023)
-
ALMS1 and Alström syndrome: a recessive form of metabolic, neurosensory and cardiac deficits
Journal of Molecular Medicine (2019)
-
Therapeutic perspectives for structural and functional abnormalities of cilia
Cellular and Molecular Life Sciences (2019)
-
Ripor2 is involved in auditory hair cell stereociliary bundle structure and orientation
Journal of Molecular Medicine (2018)
-
Cilia distribution and polarity in the epithelial lining of the mouse middle ear cavity
Scientific Reports (2017)