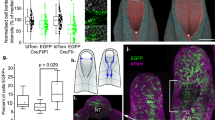
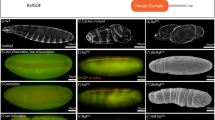
Abstract
Mice homozygous for mutations in Dact1 (also called Dapper or Frodo) phenocopy human malformations involving the spine, genitourinary system and distal digestive tract. We traced this phenotype to disrupted germ-layer morphogenesis at the primitive streak. Notably, heterozygous mutation of Vangl2, a transmembrane component of the planar cell polarity (PCP) pathway, rescued recessive Dact1 phenotypes, whereas loss of Dact1 reciprocally rescued semidominant Vangl2 phenotypes. We show that Dact1, an intracellular protein, forms a complex with Vangl2. In Dact1 mutants, Vangl2 was increased at the primitive streak, where cells ordinarily undergo an epithelial–mesenchymal transition. This is associated with abnormal E-cadherin distribution and changes in biochemical measures of the PCP pathway. We conclude that Dact1 contributes to morphogenesis at the primitive streak by regulating Vangl2 upstream of cell adhesion and the PCP pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yoshikawa, Y., Fujimori, T., McMahon, A.P. & Takada, S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev. Biol. 183, 234–242 (1997).
Torban, E. et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc. Natl. Acad. Sci. USA 105, 3449–3454 (2008).
Wang, J. et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133, 1767–1778 (2006).
Ybot-Gonzalez, P. et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134, 789–799 (2007).
Veeman, M.T., Axelrod, J.D. & Moon, R.T. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5, 367–377 (2003).
Torban, E., Wang, H.J., Groulx, N. & Gros, P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J. Biol. Chem. 279, 52703–52713 (2004).
Huang, H. & He, X. Wnt/β-catenin signaling: new (and old) players and new insights. Curr. Opin. Cell Biol. 20, 119–125 (2008).
Etheridge, S.L. et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 4, e1000259 (2008).
Zhang, L., Gao, X., Wen, J., Ning, Y. & Chen, Y.G. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J. Biol. Chem. 281, 8607–8612 (2006).
Gloy, J., Hikasa, H. & Sokol, S.Y. Frodo interacts with Dishevelled to transduce Wnt signals. Nat. Cell Biol. 4, 351–357 (2002).
Jiang, X. et al. DACT3 is an epigenetic regulator of Wnt/β-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 13, 529–541 (2008).
Cheyette, B.N.R. et al. Dapper, a Dishevelled-associated antagonist of β-catenin and JNK signaling, is required for notochord formation. Dev. Cell 2, 449–461 (2002).
Lagathu, C. et al. Dact1, a nutritionally regulated preadipocyte gene controls adipogenesis by co-ordinating the Wnt/β-catenin signalling network. Diabetes 58, 609–619 (2009).
Fisher, D.A. et al. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev. Dyn. 235, 2620–2630 (2006).
Tam, P.P. A study of the pattern of prospective somites in the presomitic mesoderm of mouse embryos. J. Embryol. Exp. Morphol. 92, 269–285 (1986).
Zhang, N. & Gridley, T. Defects in somite formation in lunatic fringe-deficient mice. Nature 394, 374–377 (1998).
Ikeya, M. & Takada, S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech. Dev. 103, 27–33 (2001).
Montcouquiol, M. et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J. Neurosci. 26, 5265–5275 (2006).
Echelard, Y. et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993).
Aulehla, A. et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell 4, 395–406 (2003).
Lickert, H. et al. Dissecting Wnt/β-catenin signaling during gastrulation using RNA interference in mouse embryos. Development 132, 2599–2609 (2005).
Staal, F.J., Noort, M.v., Strous, G.J. & Clevers, H.C. Wnt signals are transmitted through N-terminally dephosphorylated β-catenin. EMBO Rep. 3, 63–68 (2002).
Park, J.I. et al. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev. Cell 11, 683–695 (2006).
Vandenberg, A.L. & Sassoon, D.A. Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development 136, 1559–1570 (2009).
Feng, J. et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 274, 37385–37390 (1999).
Hibi, M., Lin, A., Smeal, T., Minden, A. & Karin, M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 7, 2135–2148 (1993).
Kibar, Z. et al. Identification of a new chemically induced allele (Lp(m1Jus)) at the loop-tail locus: morphology, histology, and genetic mapping. Genomics 72, 331–337 (2001).
Park, M. & Moon, R.T. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 4, 20–25 (2002).
Kibar, Z. et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28, 251–255 (2001).
Le Douarin, N.M., Teillet, M.A. & Catala, M. Neurulation in amniote vertebrates: a novel view deduced from the use of quail-chick chimeras. Int. J. Dev. Biol. 42, 909–916 (1998).
Hunter, N.L., Hikasa, H., Dymecki, S.M. & Sokol, S.Y. Vertebrate homologues of Frodo are dynamically expressed during embryonic development in tissues undergoing extensive morphogenetic movements. Dev. Dyn. 235, 279–284 (2006).
Torban, E. et al. Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr. Patterns 7, 346–354 (2007).
Devenport, D. & Fuchs, E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell Biol. 10, 1257–1268 (2008).
Pauli, R.M. Lower mesodermal defects: a common cause of fetal and early neonatal death. Am. J. Med. Genet. 50, 154–172 (1994).
Stevenson, R.E. et al. Vascular steal: the pathogenetic mechanism producing sirenomelia and associated defects of the viscera and soft tissues. Pediatrics 78, 451–457 (1986).
Manzoni, G.A., Ransley, P.G. & Hurwitz, R.S. Cloacal exstrophy and cloacal exstrophy variants: a proposed system of classification. J. Urol. 138, 1065–1068 (1987).
Duesterhoeft, S.M., Ernst, L.M., Siebert, J.R. & Kapur, R.P. Five cases of caudal regression with an aberrant abdominal umbilical artery: further support for a caudal regression-sirenomelia spectrum. Am. J. Med. Genet. A. 143A, 3175–3184 (2007).
Ciruna, B. & Rossant, J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1, 37–49 (2001).
Nelson, W.J. & Nusse, R. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 (2004).
Suriben, R., Fisher, D.A. & Cheyette, B.N. Dact1 presomitic mesoderm expression oscillates in phase with Axin2 in the somitogenesis clock of mice. Dev. Dyn. 235, 3177–3183 (2006).
Takeuchi, M. et al. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 13, 674–679 (2003).
Park, E., Kim, G.H., Choi, S.C. & Han, J.K. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev. Biol. 292, 344–357 (2006).
Veeman, M.T., Slusarski, D.C., Kaykas, A., Louie, S.H. & Moon, R.T. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 13, 680–685 (2003).
Yan, D. et al. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc. Natl. Acad. Sci. USA 98, 3802–3807 (2001).
Phillips, H.M. et al. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ. Res. 101, 137–145 (2007).
Hoch, R.V. & Soriano, P. Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development 133, 663–673 (2006).
Hyenne, V. et al. Vezatin, a protein associated to adherens junctions, is required for mouse blastocyst morphogenesis. Dev. Biol. 287, 180–191 (2005).
Angers, S. et al. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 (2006).
Klingensmith, J. et al. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech. Dev. 58, 15–26 (1996).
Copp, A.J., Checiu, I. & Henson, J.N. Developmental basis of severe neural tube defects in the loop-tail (Lp) mutant mouse: use of microsatellite DNA markers to identify embryonic genotype. Dev. Biol. 165, 20–29 (1994).
Acknowledgements
We thank all members of the Cheyette laboratory for thoughtful input and assistance with mouse husbandry and genetics. E.P. Fox provided technical expertise for creation of the Dact1 targeting construct, starting from the pGKneoF2L2DTA2 vector donated by P. Soriano (Fred Hutchinson Cancer Research Center). Embryonic stem cell manipulation and chimera production were accomplished at the University of Washington Transgenic Resources Program. U. Grieshammer provided expert assistance with neonatal genitourinary phenotyping. Confocal microscopy was accomplished in the Nikon Imaging Center of the California Institute for Quantitative Biosciences at the University of California, San Francisco, with advice from K. Thorn. S. Dymecki (Harvard Medical School) provided the Tg(ACTFLPe)9205Dym transgenic mouse line, A. McMahon (Harvard University) the Wnt3atm1Amc mutant mouse line and S. Piccolo (University of Padua) the Tg(BAT-LacZ)3Picc line. T. Gridley (The Jackson Laboratory) provided probes for Uncx and Dll1. M. Montcouquiol (INSERM/Université Bourdeaux II) provided affinity-purified Vangl2 antibody. A. Wynshaw-Boris provided helpful comments on the final manuscript. B.N.R.C. gratefully acknowledges support from US National Institutes of Health K08MH001750 and R01HD055300, the Sandler Foundation, the UCSF Academic Senate Committee on Research and the UCSF Center for Neurobiology and Psychiatry. R.S. is a recipient of a predoctoral fellowship from the California Institute of Regenerative Medicine. D.A.C.F. received support from the Medical Scientist Training Program at Washington University St. Louis.
Author information
Authors and Affiliations
Contributions
R.S. contributed gene expression, QPCR (Wnt signaling targets), most embryological analyses, confocal microscopy and genetics. S.K. contributed antigen synthesis, antibody characterization, protein blots, kinase assays, coIPs, pulldowns and associated plasmid constructs. D.A.C.F. contributed skeletal and somitogenic analyses, BAT-gal WISH and genetics including the initial Vangl2Lp cross. B.N.R.C. designed the Dact1 targeting construct in the laboratory of R.T.M. at the University of Washington. The experiments of R.S., S.K. and D.A.C.F. were performed at UCSF in the laboratory of B.N.R.C., who supervised this research and wrote the text with feedback from all authors.
Corresponding author
Ethics declarations
Competing interests
The Dact1 antibody described in this article, which was made in the laboratory of B.N.R.C., is being licensed by the University of California, San Francisco, to AbD Serotec for distribution.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–4 (PDF 1073 kb)
Rights and permissions
About this article
Cite this article
Suriben, R., Kivimäe, S., Fisher, D. et al. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet 41, 977–985 (2009). https://doi.org/10.1038/ng.435
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.435
This article is cited by
-
Heterozygous variants in the DVL2 interaction region of DACT1 cause CAKUT and features of Townes–Brocks syndrome 2
Human Genetics (2023)
-
USP39 is essential for mammalian epithelial morphogenesis through upregulation of planar cell polarity components
Communications Biology (2022)
-
Vangl2 participates in the primary ciliary assembly under low fluid shear stress in hUVECs
Cell and Tissue Research (2022)
-
Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure
Cellular and Molecular Life Sciences (2022)
-
DACT2 is a functional tumor suppressor through inhibiting Wnt/β-catenin pathway and associated with poor survival in colon cancer
Oncogene (2015)