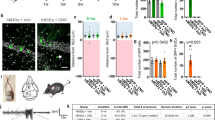
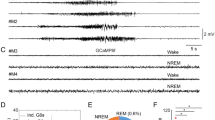
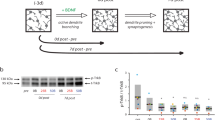
Abstract
Excitotoxicity is a process in which glutamate or other excitatory amino acids induce neuronal cell death. Accumulating evidence suggests that excitotoxicity may contribute to human neuronal cell loss caused by acute insults and chronic degeneration in the central nervous system1,2,3,4. The immediate early gene (IEG) c-fos encodes a transcription factor5,6. The c-Fos proteins form heterodimers with Jun family proteins, and the resulting AP-1 complexes regulate transcription by binding to the AP-1 sequence found in many cellular genes7,8,9. Emerging evidence suggests that c-fos is essential in regulating neuronal cell survival versus death10. Although c-fos is induced by neuronal activity, including kainic acid-induced seizures11,12,13,14, whether and how c-fos is involved in excitotoxicity is still unknown. To address this issue, we generated a mouse in which c-fos expression is largely eliminated in the hippocampus. We found that these mutant mice have more severe kainic acid–induced seizures, increased neuronal excitability and neuronal cell death, compared with control mice. Moreover, c-Fos regulates the expression of the kainic acid receptor GluR6 and brain-derived neurotrophic factor (BDNF), both in vivo and in vitro. Our results suggest that c-fos is a genetic regulator for cellular mechanisms mediating neuronal excitability and survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1, 623–634 (1988).
Coyle, J.T. & Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 262, 689–695 (1993).
Lee, J.-M., Zipfel, G.J. & Choi, D.W. The changing landscape of ischaemic brain injury mechanisms. Nature 399, A7–A14 (1999).
McNamara, J.O. Emerging insights into the genesis of epilepsy. Nature 399, A15–A22 (1999).
Muller, R., Tremblay, J.M., Adamson, E.D. & Verma, I.M. Tissue and cell type-specific expression of two human c-onc genes. Nature 304, 454–456 (1983).
Greenberg, M.E. & Ziff, E.M. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311, 433–438 (1984).
Chiu, R. et al. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 54, 541–542 (1988).
Halazonetis, T.D., Georgopoulos, K., Greenberg, M.E. & Leder, P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 55, 917–924 (1988).
Kouzarides, T. & Ziff, E. The role of the leucine zipper in the Fos-Jun interaction. Nature 336, 646–651 (1988).
Smeyne, R.J. et al. Continuous c-fos expression precedes programmed cell death in vivo. Nature 363, 166–169 (1993).
Popovici, T. et al. Effects of kainic acid-induced seizures and ischemia on c-fos-like proteins in rat brain. Brain Res. 536, 183–194 (1990).
Smeyne, R.J. et al. Fos-lacZ transgenic mice: mapping sites of gene induction in the central nervous system. Neuron 8, 13–23 (1992).
Ferkany, J.W., Zaczek, R. & Coyle, J.T. The mechanism of kainic acid neurotoxicity. Nature 308, 561–562 (1984).
Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal epilepsy. Neuroscience 14, 375–403 (1985).
Tsien, J.Z. et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326 (1996).
Yang, D.D. et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865–870 (1997).
Medvedev, A., Mackenzie, L., Hiscock, J.J. & Willoughby, J.O. Kainic acid induces distinct types of epileptiform discharge with differential involvement of hippocampus and neocortex. Brain Res. Bull. 52, 89–98 (2000).
Nadler, J.V., Perry, B.W. & Cotman, C.W. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature 271, 676–677 (1978).
Mandelzys, A., Gruda, M.A., Bravo, R. & Morgan, J.I. Absence of a persistently elevated 37 kDa fos-related antigen and AP-1-like DNA-binding activity in the brains of kainic acid-treated fosB null mice. J. Neurosci. 17, 5407–5415 (1997).
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Ann. Rev. Neurosci. 17, 31–108 (1994).
Koh, J.-Y., Gwag, B.J., Lobner, D. & Choi, D.W. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science 268, 573–575 (1995).
Tandon, P., Yang, Y., Das, K., Holmes, G.L. & Stafstrom, C.E. Neuroprotective effects of brain-derived neurotrophic factor in seizures during development. Neuroscience 91, 293–303 (1999).
Gall, C.M. Seizure-induced changes in neurotrophin expression: implications for epilepsy. Exp. Neurol. 124, 150–166 (1993).
Merlio, J.-P. et al. Increased production of the TrkB protein tyrosine kinase receptor after brain insults. Neuron 10, 151–164 (1993).
Roux, P.P., Colicos, M.A., Barker, P.A. & Kennedy, T.E. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J. Neurosci. 19, 6887–6896 (1999).
Mulle, C. et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392, 601–605 (1998).
Xu, M. et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79, 729–742 (1994).
Acknowledgements
We are grateful to J. Duffy and M. Yin for mouse blastocyst injections. We thank L. Chen, R. Hennigan, H. Jansen, A. Kuan, H. Lee, D. Lou, M. Privitera, L. Sherman and R. Walsh for various advice, help and discussions. J.Z. and M.X. are supported by grants from the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression and the Epilepsy Foundation of America. J.S.M. is partially supported by an NIH predoctoral training grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, J., Zhang, D., McQuade, J. et al. c-fos regulates neuronal excitability and survival. Nat Genet 30, 416–420 (2002). https://doi.org/10.1038/ng859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng859
This article is cited by
-
Photoreceptors inhibit pathological retinal angiogenesis through transcriptional regulation of Adam17 via c-Fos
Angiogenesis (2024)
-
Sericin alleviates motor dysfunction by modulating inflammation and TrkB/BDNF signaling pathway in the rotenone-induced Parkinson’s disease model
BMC Pharmacology and Toxicology (2023)
-
Post-Weaning Treatment with Probiotic Inhibited Stress-Induced Amnesia in Adulthood Rats: The Mediation of GABAergic System and BDNF/c-Fos Signaling Pathways
Neurochemical Research (2022)
-
Peripheral viral challenge increases c-fos level in cerebral neurons
Metabolic Brain Disease (2021)
-
Modulation of mTOR and CREB pathways following mGluR5 blockade contribute to improved Huntington’s pathology in zQ175 mice
Molecular Brain (2019)