Abstract
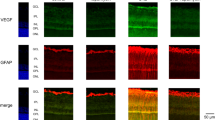
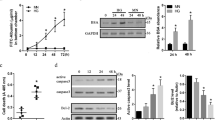
Cellular apoptosis induced by hyperglycemia occurs in many vascular cells and is crucial for the initiation of diabetic pathologies. In the retina, pericyte apoptosis and the formation of acellular capillaries, the most specific vascular pathologies attributed to hyperglycemia, is linked to the loss of platelet-derived growth factor (PDGF)-mediated survival actions owing to unknown mechanisms. Here we show that hyperglycemia persistently activates protein kinase C-δ (PKC-δ, encoded by Prkcd) and p38α mitogen-activated protein kinase (MAPK) to increase the expression of a previously unknown target of PKC-δ signaling, Src homology-2 domain–containing phosphatase-1 (SHP-1), a protein tyrosine phosphatase. This signaling cascade leads to PDGF receptor-β dephosphorylation and a reduction in downstream signaling from this receptor, resulting in pericyte apoptosis independently of nuclear factor-κB (NF-κB) signaling. We observed increased PKC-δ activity and an increase in the number of acellular capillaries in diabetic mouse retinas, which were not reversible with insulin treatment that achieved normoglycemia. Unlike diabetic age-matched wild-type mice, diabetic Prkcd−/− mice did not show activation of p38α MAPK or SHP-1, inhibition of PDGF signaling in vascular cells or the presence of acellular capillaries. We also observed PKC-δ, p38α MAPK and SHP-1 activation in brain pericytes and in the renal cortex of diabetic mice. These findings elucidate a new signaling pathway by which hyperglycemia can induce PDGF resistance and increase vascular cell apoptosis to cause diabetic vascular complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lorenzi, M. & Gerhardinger, C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia 44, 791–804 (2001).
Isermann, B. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 13, 1349–1358 (2007).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
Mizutani, M., Kern, T.S. & Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Invest. 97, 2883–2890 (1996).
Busik, J.V., Mohr, S. & Grant, M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57, 1952–1965 (2008).
Hammes, H.P. Pericytes and the pathogenesis of diabetic retinopathy. Horm. Metab. Res. 37 Suppl 1, 39–43 (2005).
Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. J. Am. Med. Assoc. 290, 2159–2167 (2003).
Kuwabara, T. & Cogan, D.G. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch. Ophthalmol. 69, 492–502 (1963).
Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 350, 48–58 (2004).
Enge, M. et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 21, 4307–4316 (2002).
Yokota, T. et al. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes 52, 838–845 (2003).
Koya, D. & King, G.L. Protein kinase C activation and the development of diabetic complications. Diabetes 47, 859–866 (1998).
Ryer, E.J. et al. Protein kinase C δ induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J. Biol. Chem. 280, 35310–35317 (2005).
Park, J.Y. et al. Induction of endothelin-1 expression by glucose: an effect of protein kinase C activation. Diabetes 49, 1239–1248 (2000).
Igarashi, M. et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J. Clin. Invest. 103, 185–195 (1999).
Zarubin, T. & Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 (2005).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Yu, Z. et al. SHP-1 associates with both platelet-derived growth factor receptor and the p85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem. 273, 3687–3694 (1998).
Nakase, K., Cheng, J., Zhu, Q. & Marasco, W.A. Mechanisms of SHP-1 P2 promoter regulation in hematopoietic cells and its silencing in HTLV-1-transformed T cells. J. Leukoc. Biol. 85, 165–174 (2009).
Romeo, G., Liu, W.H., Asnaghi, V., Kern, T.S. & Lorenzi, M. Activation of nuclear factor-κB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 51, 2241–2248 (2002).
Shoelson, S.E., Lee, J. & Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Suzuma, K. et al. Characterization of protein kinase C beta isoform's action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc. Natl. Acad. Sci. USA 99, 721–726 (2002).
Idris, I., Gray, S. & Donnelly, R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia 44, 659–673 (2001).
Ishii, H. et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 272, 728–731 (1996).
Aiello, L.P. et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 331, 1480–1487 (1994).
The PKC-diabetic retinopathyS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C β Inhibitor Diabetic Retinopathy Study (PKC-diabetic retinopathyS) multicenter randomized clinical trial. Diabetes 54, 2188–2197 (2005).
Kanthasamy, A.G., Kitazawa, M., Kanthasamy, A. & Anantharam, V. Role of proteolytic activation of protein kinase Cδ in oxidative stress–induced apoptosis. Antioxid. Redox Signal. 5, 609–620 (2003).
Miao, F. et al. Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J. Biol. Chem. 282, 13854–13863 (2007).
El-Osta, A. et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 205, 2409–2417 (2008).
Villeneuve, L.M. et al. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. USA 105, 9047–9052 (2008).
Peter, P.S. et al. Inhibition of p38 α MAPK rescues cardiomyopathy induced by overexpressed β2-adrenergic receptor, but not β1-adrenergic receptor. J. Clin. Invest. 117, 1335–1343 (2007).
Nemoto, S., Xiang, J., Huang, S. & Lin, A. Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. J. Biol. Chem. 273, 16415–16420 (1998).
Dubois, M.J. et al. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat. Med. 12, 549–556 (2006).
Tenev, T. et al. Both SH2 domains are involved in interaction of SHP-1 with the epidermal growth factor receptor but cannot confer receptor-directed activity to SHP-1/SHP-2 chimera. J. Biol. Chem. 272, 5966–5973 (1997).
Bhattacharya, R., Kwon, J., Wang, E., Mukherjee, P. & Mukhopadhyay, D. Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF receptor-2 and attenuates endothelial DNA synthesis, but not migration*. J. Mol. Signal. 3, 8 (2008).
Shultz, L.D. et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 73, 1445–1454 (1993).
Bignon, J.S. & Siminovitch, K.A. Identification of PTP1C mutation as the genetic defect in motheaten and viable motheaten mice: a step toward defining the roles of protein tyrosine phosphatases in the regulation of hemopoietic cell differentiation and function. Clin. Immunol. Immunopathol. 73, 168–179 (1994).
Lyons, B.L. et al. Deficiency of SHP-1 protein-tyrosine phosphatase in “viable motheaten” mice results in retinal degeneration. Invest. Ophthalmol. Vis. Sci. 47, 1201–1209 (2006).
Leitges, M. et al. Exacerbated vein graft arteriosclerosis in protein kinase Cδ-null mice. J. Clin. Invest. 108, 1505–1512 (2001).
Kern, T.S. & Engerman, R.L. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr. Eye Res. 13, 863–867 (1994).
King, G.L., Berman, A.B., Bonner-Weir, S. & Carson, M.P. Regulation of vascular permeability in cell culture. Diabetes 36, 1460–1467 (1987).
Lindahl, P., Johansson, B.R., Leveen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B–deficient mice. Science 277, 242–245 (1997).
Kanwar, M., Chan, P.S., Kern, T.S. & Kowluru, R.A. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest. Ophthalmol. Vis. Sci. 48, 3805–3811 (2007).
Wang, Y. et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273, 2161–2168 (1998).
Acknowledgements
Prkcd−/− mice were provided by M. Leitges (University of Oslo). Adenoviral vectors expressing dominant-negative p38α and p38β MAPK were generously provided by Y. Wang (University of California, Los Angeles). Adenoviral vector expressing dominant-negative of SHP-1 (Ad-DN SHP-1) was generously provided by A. Marette (University of Laval). P.G. is a recipient of awards from the Juvenile Diabetes Research Foundation. This study was supported by the US National Eye Institute (grant 5R01EY016150-02to G.L.K.) and by the Canadian Institute of Health Research (grant 165453) to A.M. We thank W.-C. Li and S. Bonner-Weir for the technical support on confocal microscopy imaging.
Author information
Authors and Affiliations
Contributions
P.G. and G.L.K. conceived of, designed and performed most of the research, analyzed the data and wrote the manuscript. J.H.-Y. and M.M. conducted the research experiments using the laser microdissection and rat studies. A.C. performed the RBF and MCT studies. M.L. provided the Prkcd−/− mice. A.M. provided the adenoviral vector encoding the dominant-negative form of SHP-1. L.P.A. conceived of and edited the manuscript. T.S.K. performed the acellular capillary and pericyte loss measurements, analyzed the data and edited the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–9, Supplementary Table 1 and Supplementary Methods (PDF 1209 kb)
Rights and permissions
About this article
Cite this article
Geraldes, P., Hiraoka-Yamamoto, J., Matsumoto, M. et al. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 15, 1298–1306 (2009). https://doi.org/10.1038/nm.2052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2052
This article is cited by
-
Research progress on the pathogenesis of diabetic retinopathy
BMC Ophthalmology (2023)
-
Oxidative stress and epigenetics in ocular vascular aging: an updated review
Molecular Medicine (2023)
-
PKCδ regulates the vascular biology in diabetic atherosclerosis
Cell Communication and Signaling (2023)
-
Diabetic vascular diseases: molecular mechanisms and therapeutic strategies
Signal Transduction and Targeted Therapy (2023)
-
Qualitative and quantitative evaluation of diabetic choroidopathy using ultra-widefield indocyanine green angiography
Scientific Reports (2023)