Abstract
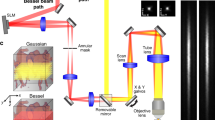
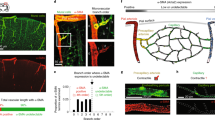
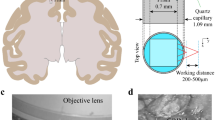
Two-photon phosphorescence lifetime microscopy (2PLM) has been used recently for depth measurements of oxygen partial pressure (PO2) in the rodent brain. In capillaries of olfactory bulb glomeruli, 2PLM has also allowed simultaneous measurements of PO2 and blood flow and revealed the presence of erythrocyte-associated transients (EATs), which are PO2 gradients that are associated with individual erythrocytes. We investigated the extent to which EAT properties in capillaries report local neuronal activity. We find that at rest, PO2 at EAT peaks overestimates the mean PO2 by 35 mm Hg. PO2 between two EAT peaks is at equilibrium with, and thus reports, PO2 in the neuropil. During odor stimulation, there is a small PO2 decrease before functional hyperemia, showing that the initial dip in PO2 is present at the level of capillaries. We conclude that imaging oxygen dynamics in capillaries provides a unique and noninvasive approach to map neuronal activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hellums, J.D. The resistance to oxygen transport in the capillaries relative to that in the surrounding tissue. Microvasc. Res. 13, 131–136 (1977).
Golub, A.S. & Pittman, R.N. Erythrocyte-associated transients in PO2 revealed in capillaries of rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 288, H2735–H2743 (2005).
Tsai, A.G. et al. Effect of oxygen consumption by measuring method on PO2 transients associated with the passage of erythrocytes in capillaries of rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 289, H1777–H1779 (2005).
Golub, A.S. & Pittman, R.N. PO2 measurements in the microcirculation using phosphorescence quenching microscopy at high magnification. Am. J. Physiol. Heart Circ. Physiol. 294, H2905–H2916 (2008).
Raichle, M.E. & Mintun, M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 29, 449–476 (2006).
Lecoq, J. et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat. Med. 17, 893–898 (2011).
Homer, L.D., Weathersby, P.K. & Kiesow, L.A. Oxygen gradients between red blood cells in the microcirculation. Microvasc. Res. 22, 308–323 (1981).
Federspiel, W.J. & Popel, A.S. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc. Res. 32, 164–189 (1986).
Groebe, K. & Thews, G. Effects of red cell spacing and red cell movement upon oxygen release under conditions of maximally working skeletal muscle. Adv. Exp. Med. Biol. 248, 175–185 (1989).
Wang, C.H. & Popel, A.S. Effect of red blood cell shape on oxygen transport in capillaries. Math. Biosci. 116, 89–110 (1993).
Shepherd, G.M. & Charpak, S. The olfactory glomerulus: a model for neuro-glio-vascular biology. Neuron 58, 827–829 (2008).
Lecoq, J. et al. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J. Neurosci. 29, 1424–1433 (2009).
Chaigneau, E., Oheim, M., Audinat, E. & Charpak, S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc. Natl. Acad. Sci. USA 100, 13081–13086 (2003).
Hudetz, A.G. Blood flow in the cerebral capillary network: a review emphasizing observations with intravital microscopy. Microcirculation 4, 233–252 (1997).
Pittman, R.N. Oxygen gradients in the microcirculation. Acta Physiol. (Oxf.) 202, 311–322 (2011).
Lindauer, U. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J. Cereb. Blood Flow Metab. 30, 757–768 (2010).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Franceschini, M.A. et al. The effect of different anesthetics on neurovascular coupling 2. Neuroimage 51, 1367–1377 (2010).
Rinberg, D., Koulakov, A. & Gelperin, A. Sparse odor coding in awake behaving mice. J. Neurosci. 26, 8857–8865 (2006).
Livneh, Y. & Mizrahi, A. Experience-dependent plasticity of mature adult-born neurons. Nat. Neurosci. 15, 26–28 (2012).
Thompson, J.K., Peterson, M.R. & Freeman, R.D. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science 299, 1070–1072 (2003).
Weber, B. et al. Optical imaging of the spatiotemporal dynamics of cerebral blood flow and oxidative metabolism in the rat barrel cortex 1. Eur. J. Neurosci. 20, 2664–2670 (2004).
Devor, A. et al. “Overshoot” of O is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J. Neurosci. 31, 13676–13681 (2011).
Thompson, J.K., Peterson, M.R. & Freeman, R.D. High-resolution neurometabolic coupling revealed by focal activation of visual neurons. Nat. Neurosci. 7, 919–920 (2004).
Offenhauser, N., Thomsen, K., Caesar, K. & Lauritzen, M. Activity induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J. Physiol. (Lond.) 565, 279–294 (2005).
Frostig, R.D., Lieke, E.E., Ts'o, D.Y. & Grinvald, A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc. Natl. Acad. Sci. USA 87, 6082–6086 (1990).
Malonek, D. & Grinvald, A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272, 551–554 (1996).
Ernst, T. & Hennig, J. Observation of a fast response in functional MR. Magn. Reson. Med. 32, 146–149 (1994).
Menon, R.S. et al. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn. Reson. Med. 33, 453–459 (1995).
Kim, D.S., Duong, T.Q. & Kim, S.G. High-resolution mapping of iso-orientation columns by fMRI. Nat. Neurosci. 3, 164–169 (2000).
Silva, A.C., Lee, S.P., Iadecola, C. & Kim, S.G. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J. Cereb. Blood Flow Metab. 20, 201–206 (2000).
Lindauer, U. et al. No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation. Neuroimage 13, 988–1001 (2001).
Jones, M., Berwick, J., Johnston, D. & Mayhew, J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage 13, 1002–1015 (2001).
Buxton, R.B. The elusive initial dip. Neuroimage 13, 953–958 (2001).
Díez-García, J. et al. Activation of cerebellar parallel fibers monitored in transgenic mice expressing a fluorescent Ca2+ indicator protein. Eur. J. Neurosci. 22, 627–635 (2005).
Wachowiak, M. & Cohen, L.B. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32, 723–735 (2001).
Acknowledgements
We thank S. Vinogradov (Department of Biochemistry and Biophysics, University of Pennsylvania) for providing PtP-C343 through the intermediate of Oxygen Enterprises. We also thank G. Bouchery and A. Virolle for help in animal surgery, S. Sasnouski for software improvements and M. Ducros, B. Weber and U. Lindauer for critical comments. Support was provided by INSERM, CNRS, the Leducq Foundation, the Human Frontier Science Program Organization (HSFPO), the Agence Nationale de la Recherche and the Fondation pour la Recherche Médicale.
Author information
Authors and Affiliations
Contributions
A.P., Y.G.H. and S.C. conducted the experiments and analyzed the data. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Methods (PDF 308 kb)
Rights and permissions
About this article
Cite this article
Parpaleix, A., Houssen, Y. & Charpak, S. Imaging local neuronal activity by monitoring PO2 transients in capillaries. Nat Med 19, 241–246 (2013). https://doi.org/10.1038/nm.3059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3059
This article is cited by
-
Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: evidence for microvascular injury in the cerebral white matter
GeroScience (2023)
-
Network-driven anomalous transport is a fundamental component of brain microvascular dysfunction
Nature Communications (2021)
-
Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences
Nature Communications (2021)
-
In vivo O2 imaging in hepatic tissues by phosphorescence lifetime imaging microscopy using Ir(III) complexes as intracellular probes
Scientific Reports (2020)
-
Cerebral oxygenation during locomotion is modulated by respiration
Nature Communications (2019)