Abstract
Alzheimer's disease is associated with the intraparenchymal growth of plaque-like amyloid deposits1–3. Amyloid plaques are formed by the progressive deposition and transformation of soluble amyloid β-protein monomers into insoluble and fibrillar aggregates that contain amyloid β-protein in a β-pleated sheet conformation. This process is described as ‘seeded polymerization’ of the monomers with slow-nucleation and fast-growth kinetics4. Soluble amyloid β-protein monomers are present in the cortical extracellular space and in the cerebrospinal fluid5,6, whereas insoluble aggregates so far can be found only by the examination of brain tissue by biopsy or autopsy. Here we present a biophysical method that uses the principle of seeded polymerization in combination with fluorescence correlation spectroscopy, which allowed us to detect single amyloid β-peptide aggregates in the cerebrospinal fluid samples from Alzheimer's patients. All of 15 Alzheimer's samples but none of the 19 age-matched control samples produced large peaks with fluorescence correlation spectroscopy indicating the rapid aggregation of the fluorescent labelled synthetic amyloid β-protein probe onto the amyloid β-protein ‘seeds’ present in the cerebrospinal fluid. Our method could enable easy in vivo detection of the cerebral amyloid β-protein pathology of Alzheimer's disease and might be of potential value to facilitate its routine diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Beyreuther, K. et al. Regulation and expression of the Alzheimer's beta/A4 amyloid protein precursor in health, disease, and Down's syndrome. Ann. NY Acad. Sci. 695, 91–102 (1993).
Selkoe, D.J. Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer's disease. Annu. Rev. Cell. Biol. 10, 373–403 (1994).
Sisodia, S.S. & Price, D.L. Role of the beta-amyloid protein in Alzheimers's disease. FASEB J. 9, 366–370 (1995).
Harper, J.D. & Lansbury, P.T. Jr., Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66, 385–407 (1997).
Seubert, P. et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature 359, 325–327 (1992).
Vigo-Pelfrey, C., Lee, D., Keim, P., Lieberburg, I. & Schenk, D.B. Characterization of beta-amyloid peptide from human cerebrospinal fluid. J. Neurochem. 61, 1965–1968 (1993).
Prusiner, S.B. Prion diseases and the BSE crisis. Science 278, 245–251 (1997).
Eigen, M. & Rigler, R. Sorting single molecules: application to diagnostics and evolutionary biotechnology. Proc. Natl. Acad. Sci USA 91, 5740–5747 (1994).
Hilbich, C., Kisters-Woike, B., Reed, J., Masters, C.L. & Beyreuther, K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer's disease. J. Mol. Biol. 218, 149–163 (1991).
Terzi, E., Holzemann, G. & Seelig, J. Self-association of beta-amyloid peptide (1–40) in solution and binding to lipid membranes. J. Mol. Biol. 252, 633–642 (1995).
Tomski, J. & Murphy, R.M. Kinetics of aggregation of synthetic beta-amyloid peptide. Arch. Biochem. Biophys. 294, 630–638 (1992).
Lorenzo, A. & Yankner, B.A. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. USA 91, 12243–12247 (1994).
Bush, A.I. et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265, 1464–1467 (1994).
Brown, A.M. et al. Selective aggregation of endogenous beta-amyloid peptide and soluble amyloid precursor protein in cerebrospinal fluid by zinc. J. Neurochem. 69, 1204–1212 (1997).
Podlisny, M.B. et al. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J. Biol. Chem. 270, 9564–9570 (1998).
Motter, R. et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann. Neurol. 38, 643–648 (1995).
Nitsch, R.M. et al. Cerebrospinal fluid levels of amyloid beta-protein in Alzheimer's disease inverse correlation with severity of dementia and effect of apolipoprotein E genotype. Ann Neurol. 37, 512–518 (1995).
Dilling, H., Mombow, W., Schmidt, M.H. & Schulte-Markwort, E. (Eds.) WHO Internationale Klassifikation psychischer Störungen, ICD-10, Hans-Huber Verlag , 1994.
Reisberg, B., Ferris, S.H., De Leon, M.J. & Crook, T. The global Deterioration Scale for assessment of primary degenerative dementia: Am. J. Psychiatr. 139, 1136–1139 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pitschke, M., Prior, R., Haupt, M. et al. Detection of single amyloid β-protein aggregates in the cerebrospinal fluid of Alzheimer's patients by fluorescence correlation spectroscopy. Nat Med 4, 832–834 (1998). https://doi.org/10.1038/nm0798-832
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0798-832
This article is cited by
-
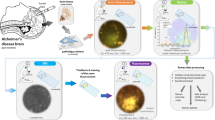
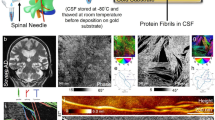
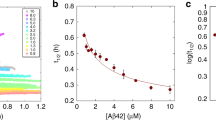
Protein fibril length in cerebrospinal fluid is increased in Alzheimer’s disease
Communications Biology (2023)
-
Amyloid β structural polymorphism, associated toxicity and therapeutic strategies
Cellular and Molecular Life Sciences (2021)
-
Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases
Nature Neuroscience (2018)
-
Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles
Nature Chemistry (2017)
-
Switchable sensitizers stepwise lighting up lanthanide emissions
Scientific Reports (2015)