Abstract
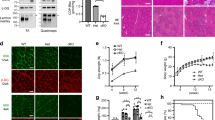
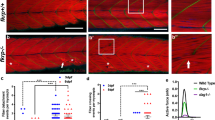
Several congenital muscular dystrophies caused by defects in known or putative glycosyltransferases are commonly associated with hypoglycosylation of α-dystroglycan (α-DG) and a marked reduction of its receptor function. We have investigated changes in the processing and function of α-DG resulting from genetic manipulation of LARGE, the putative glycosyltransferase mutated both in Largemyd mice and in humans with congenital muscular dystrophy 1D (MDC1D). Here we show that overexpression of LARGE ameliorates the dystrophic phenotype of Largemyd mice and induces the synthesis of glycan-enriched α-DG with high affinity for extracellular ligands. Notably, LARGE circumvents the α-DG glycosylation defect in cells from individuals with genetically distinct types of congenital muscular dystrophy. Gene transfer of LARGE into the cells of individuals with congenital muscular dystrophies restores α-DG receptor function, whereby glycan-enriched α-DG coordinates the organization of laminin on the cell surface. Our findings indicate that modulation of LARGE expression or activity is a viable therapeutic strategy for glycosyltransferase-deficient congenital muscular dystrophies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dubowitz, V. Congenital muscular dystrophy: an expanding clinical syndrome. Ann. Neurol. 47, 143–144 (2000).
Toda, T., Kobayashi, K., Kondo-Iida, E., Sasaki, J. & Nakamura, Y. The Fukuyama congenital muscular dystrophy story. Neuromuscul. Disord. 10, 153–159 (2000).
Dobyns, W.B. et al. Diagnostic criteria for Walker-Warburg syndrome. Am. J. Med. Genet. 32, 195–210 (1989).
Yoshida, A. et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell 1, 717–724 (2001).
Beltran-Valero De Bernabe, D. et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am. J. Hum. Genet. 71, 1033–1043 (2002).
Taniguchi, K. et al. Worldwide distribution and broader clinical spectrum of muscle-eye-brain disease. Hum. Mol. Genet. 12, 527–534 (2003).
Hayashi, Y.K. et al. Selective deficiency of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57, 115–121 (2001).
Kano, H. et al. Deficiency of α-dystroglycan in muscle-eye-brain disease. Biochem. Biophys. Res. Commun. 291, 1283–1286 (2002).
Jimenez-Mallebrera, C. et al. Profound skeletal muscle depletion of α-dystroglycan in Walker-Warburg syndrome. Eur. J. Paediatr. Neurol. 7, 129–137 (2003).
Ervasti, J.M. & Campbell, K.P. Membrane organization of the dystrophin-glycoprotein complex. Cell 66, 1121–1131 (1991).
Holt, K.H., Crosbie, R.H., Venzke, D.P. & Campbell, K.P. Biosynthesis of dystroglycan: processing of a precursor propeptide. FEBS Lett. 468, 79–83 (2000).
Ibraghimov-Beskrovnaya, O. et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 (1992).
Michele, D.E. et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417–422 (2002).
Takeda, S. et al. Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum. Mol. Genet. 12, 1449–1459 (2003).
Holzfeind, P.J. et al. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Largemyd mouse defines a natural model for glycosylation-deficient muscle-eye-brain disorders. Hum. Mol. Genet. 11, 2673–2687 (2002).
Grewal, P.K., Holzfeind, P.J., Bittner, R.E. & Hewitt, J.E. Mutant glycosyltransferase and altered glycosylation of α-dystroglycan in the myodystrophy mouse. Nat. Genet. 28, 151–154 (2001).
Longman, C. et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum. Mol. Genet. 12, 2853–2861 (2003).
Peyrard, M. et al. The human LARGE gene from 22q12.3–q13.1 is a new, distinct member of the glycosyltransferase gene family. Proc. Natl. Acad. Sci. USA 96, 598–603 (1999).
Kondo-Iida, E. et al. Novel mutations and genotype-phenotype relationships in 107 families with Fukuyama-type congenital muscular dystrophy (FCMD). Hum. Mol. Genet. 8, 2303–2309 (1999).
Cohn, R.D. et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110, 639–648 (2002).
Saito, Y., Mizuguchi, M., Oka, A. & Takashima, S. Fukutin protein is expressed in neurons of the normal developing human brain but is reduced in Fukuyama-type congenital muscular dystrophy brain. Ann. Neurol. 47, 756–764 (2000).
Zhang, W., Betel, D. & Schachter, H. Cloning and expression of a novel UDP-GlcNAc:α-D-mannoside β1,2-N-acetylglucosaminyltransferase homologous to UDP-GlcNAc:α-3-D-mannoside β1,2-N-acetylglucosaminyltransferase I. Biochem. J. 361, 153–162 (2002).
Ervasti, J.M. & Campbell, K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 122, 809–823 (1993).
Chiba, A. et al. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of α-dystroglycan with laminin. J. Biol. Chem. 272, 2156–2162 (1997).
Zhang, W. et al. Enzymatic diagnostic test for muscle-eye-brain type congenital muscular dystrophy using commercially available reagents. Clin. Biochem. 36, 339–344 (2003).
Jurado, L.A., Coloma, A. & Cruces, J. Identification of a human homolog of the Drosophila rotated abdomen gene (POMT1) encoding a putative protein O-mannosyl-transferase, and assignment to human chromosome 9q34.1. Genomics 58, 171–180 (1999).
Sabatelli, P. et al. Extracellular matrix and nuclear abnormalities in skeletal muscle of a patient with Walker-Warburg syndrome caused by POMT1 mutation. Biochim. Biophys. Acta 1638, 57–62 (2003).
Ishii, H., Hayashi, Y.K., Nonaka, I. & Arahata, K. Electron microscopic examination of basal lamina in Fukuyama congenital muscular dystrophy. Neuromuscul. Disord. 7, 191–197 (1997).
Cohen, M.W., Jacobson, C., Yurchenco, P.D., Morris, G.E. & Carbonetto, S. Laminin-induced clustering of dystroglycan on embryonic muscle cells: comparison with agrin-induced clustering. J. Cell Biol. 136, 1047–1058 (1997).
Henry, M.D. & Campbell, K.P. A role for dystroglycan in basement membrane assembly. Cell 95, 859–870 (1998).
Xia, B. et al. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev. Biol. 242, 58–73 (2002).
Nguyen, H.H., Jayasinha, V., Xia, B., Hoyte, K. & Martin, P.T. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc. Natl. Acad. Sci. USA 99, 5616–5621 (2002).
Longman, C. et al. Novel forms of muscular dystrophy with α-dystroglycan deficiency and absent CNS involvement. Neuromuscul. Disord. 13, 645 (2003).
Kunz, S., Sevilla, N., McGavern, D.B., Campbell, K.P. & Oldstone, M.B. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J. Cell Biol. 155, 301–310 (2001).
Duclos, F. et al. Progressive muscular dystrophy in α-sarcoglycan-deficient mice. J. Cell Biol. 142, 1461–1471 (1998).
Durbeej, M. et al. Gene transfer establishes primacy of striated vs. smooth muscle sarcoglycan complex in limb-girdle muscular dystrophy. Proc. Natl. Acad. Sci. USA 100, 8910–8915 (2003).
Kobayashi, K. et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394, 388–392 (1998).
Kanoff, R.J. et al. Walker-Warburg syndrome: neurologic features and muscle membrane structure. Pediatr. Neurol. 18, 76–80 (1998).
Henry, M.D. et al. Distinct roles for dystroglycan, β1 integrin and perlecan in cell surface laminin organization. J. Cell Sci. 114, 1137–1144 (2001).
Acknowledgements
We thank C.C. Chen and all members of the Campbell laboratory for discussions and technical support; J. Lilien for discussions; J. Vajsar for the MEB fibroblasts; M. Oldstone for the DGFc5 construct; and T. Südhof for the neurexin fusion protein cDNA. We also thank C. Lovig and the University of Iowa Hybridoma Facility, and the University of Iowa Gene Transfer Vector Core, which is supported in part by the Carver Foundation and the NIH. Samples used in this study were provided in part by the National Institute of Child Health and Human Development Brain Bank HD83284. This work was supported by the Muscular Dystrophy Association (R.B., S.A.M., K.P.C.) and the NIH (S.A.M.). D.E.M. was supported by a Cardiovascular Interdisciplinary Research Fellowship and a University of Iowa Biosciences Initiative Fellowship. K.P.C. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
DGC expression in Largemyd skeletal muscle after LARGE gene transfer. (PDF 175 kb)
Supplementary Fig. 2
Improved morphology and function of Largemyd transduced skeletal muscle. (PDF 209 kb)
Supplementary Fig. 3
Forced expression of POMGnT1 does not improve the functional glycosylation of α-DG. (PDF 283 kb)
Rights and permissions
About this article
Cite this article
Barresi, R., Michele, D., Kanagawa, M. et al. LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med 10, 696–703 (2004). https://doi.org/10.1038/nm1059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1059
This article is cited by
-
DAG1 haploinsufficiency is associated with sporadic and familial isolated or pauci-symptomatic hyperCKemia
European Journal of Human Genetics (2024)
-
Cell surface glycan engineering reveals that matriglycan alone can recapitulate dystroglycan binding and function
Nature Communications (2022)
-
Profiling of the muscle-specific dystroglycan interactome reveals the role of Hippo signaling in muscular dystrophy and age-dependent muscle atrophy
BMC Medicine (2020)
-
NAD+ improves neuromuscular development in a zebrafish model of FKRP-associated dystroglycanopathy
Skeletal Muscle (2019)
-
LARGE expression in different types of muscular dystrophies other than dystroglycanopathy
BMC Neurology (2018)