Abstract
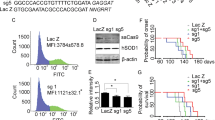
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease resulting in the selective death of motor neurons in the brain and spinal cord1. Some familial cases of ALS are caused by dominant mutations in the gene encoding superoxide dismutase (SOD1)2,3,4. The emergence of interfering RNA (RNAi) for specific gene silencing could be therapeutically beneficial for the treatment of such dominantly inherited diseases5,6,7. We generated a lentiviral vector to mediate expression of RNAi molecules specifically targeting the human SOD1 gene (SOD1). Injection of this vector into various muscle groups of mice engineered to overexpress a mutated form of human SOD1 (SOD1G93A) resulted in an efficient and specific reduction of SOD1 expression and improved survival of vulnerable motor neurons in the brainstem and spinal cord. Furthermore, SOD1 silencing mediated an improved motor performance in these animals, resulting in a considerable delay in the onset of ALS symptoms by more than 100% and an extension in survival by nearly 80% of their normal life span. These data are the first to show a substantial extension of survival in an animal model of a fatal, dominantly inherited neurodegenerative condition using RNAi and provide the highest therapeutic efficacy observed in this field to date.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mulder, D.W. Clinical limits of amyotrophic lateral sclerosis. Adv. Neurol. 36, 15–22 (1982).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Deng, H.X. et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261, 1047–1051 (1993).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Maxwell, M.M., Pasinelli, P., Kazantsev, A.G. & Brown, R.H., Jr. RNA interference-mediated silencing of mutant superoxide dismutase rescues cyclosporin A-induced death in cultured neuroblastoma cells. Proc. Natl. Acad. Sci. USA 101, 3178–3183 (2004).
Ding, H. et al. Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell 2, 209–217 (2003).
Xia, H. et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med. 8, 816–820 (2004).
Xia, H., Mao, Q., Paulson, H.L. & Davidson, B.L. siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotechnol. 20, 1006–1010 (2002).
Hommel, J.D., Sears, R.M., Georgescu, D., Simmons, D.L. & DiLeone, R.J. Local gene knockdown in the brain using viral-mediated RNA interference. Nat. Med. 9, 1539–1544 (2003).
Mazarakis, N.D. et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum. Mol. Genet. 10, 2109–2121 (2001).
Wong, L.F. et al. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol. Ther. 9, 101–111 (2004).
Azzouz, M. et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 429, 413–417 (2004).
Wong, P.C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Bruijn, L.I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Cleveland, D.W. & Rothstein, J.D. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2, 806–819 (2001).
Brummelkamp, T.R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Katsuno, M. et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002).
Katsuno, M. et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat. Med. 9, 768–773 (2003).
Adachi, H. et al. Transgenic mice with an expanded CAG repeat controlled by the human AR promoter show polyglutamine nuclear inclusions and neuronal dysfunction without neuronal cell death. Hum Mol. Genet. 10, 1039–1048 (2001).
Kaspar, B.K., Llado, J., Sherkat, N., Rothstein, J.D. & Gage, F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301, 839–842 (2003).
Clement, A.M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
Mitrophanous, K. et al. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther. 6, 1808–1818 (1999).
Soneoka, Y. et al. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23, 628–633 (1995).
Martin-Rendon, E., White, L.J., Olsen, A., Mitrophanous, K.A. & Mazarakis, N.D. New Methods to Titrate EIAV-Based Lentiviral Vectors. Mol. Ther. 5, 566–570 (2002).
Abercrombie, M. Estimation of nuclear population from microtome sections. Anatomical Record 94, 239–247 (1948).
Acknowledgements
Many thanks to the Virology and Neurobiology teams at Oxford BioMedica for assistance with design and preparation of viral vectors. L.G.B. is a Wellcome Trust Prize student. L.G. is the Graham Watts Senior Research Fellow funded by the Brain Research Trust. This work was funded by Oxford Biomedica Ltd.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors GSR, PAR, DMD, JMM, MAL, LFW, SMK, KAM, NDM, and MA are share holders in Oxford Biomedica (UK) Ltd and as such have competing financial interests in the publication of this work.
Supplementary information
Supplementary Fig. 1
Analysis of transgene copy number in EIAV-injected SOD1G93A mice used for this study. (PDF 60 kb)
Rights and permissions
About this article
Cite this article
Ralph, G., Radcliffe, P., Day, D. et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med 11, 429–433 (2005). https://doi.org/10.1038/nm1205
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1205
This article is cited by
-
Possibilities and limitations of antisense oligonucleotide therapies for the treatment of monogenic disorders
Communications Medicine (2024)
-
Amyotrophic lateral sclerosis: translating genetic discoveries into therapies
Nature Reviews Genetics (2023)
-
Advances in gene therapy for neurogenetic diseases: a brief review
Journal of Molecular Medicine (2022)
-
Non-neuronal cells in amyotrophic lateral sclerosis — from pathogenesis to biomarkers
Nature Reviews Neurology (2021)
-
The deletion of mutant SOD1 via CRISPR/Cas9/sgRNA prolongs survival in an amyotrophic lateral sclerosis mouse model
Gene Therapy (2020)