Abstract
We developed an integrated experimental framework that extends the brain exploration capabilities of functional ultrasound imaging to awake and mobile rats. In addition to acquiring hemodynamic data, this method further allows parallel access to electroencephalography (EEG) recordings of neuronal activity. We illustrate this approach with two proofs of concept: a behavioral study on theta rhythm activation in a maze running task and a disease-related study on spontaneous epileptic seizures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Logothetis, N.K. Nature 453, 869–878 (2008).
Sada, N., Lee, S., Katsu, T., Otsuki, T. & Inoue, T. Science 347, 1362–1367 (2015).
Kerr, J.N. & Nimmerjahn, A. Curr. Opin. Neurobiol. 22, 45–53 (2012).
Packer, A.M., Russell, L.E., Dalgleish, H.W. & Häusser, M. Nat. Methods 12, 140–146 (2015).
Macé, E. et al. Nat. Methods 8, 662–664 (2011).
Macé, E. et al. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 60, 492–506 (2013).
Osmanski, B.F., Pezet, S., Ricobaraza, A., Lenkei, Z. & Tanter, M. Nat. Commun. 5, 5023 (2014).
Buzsáki, G. & Moser, E.I. Nat. Neurosci. 16, 130–138 (2013).
David, O. et al. PLoS Biol. 6, 2683–2697 (2008).
Zheng, T.W. et al. Epilepsia 53, 1948–1958 (2012).
Jacobs, J. et al. Neuroimage 45, 1220–1231 (2009).
Koehler, R.C., Roman, R.J. & Harder, D.R. Trends Neurosci. 32, 160–169 (2009).
LaMendola, N.P. & Bever, T.G. Science 278, 483–486 (1997).
Logothetis, N.K. et al. Nature 491, 547–553 (2012).
Mishra, A.M. et al. J. Neurosci. 31, 15053–15064 (2011).
Roche-Labarbe, N. et al. Epilepsia 51, 1374–1384 (2010).
O'Neill, P.K., Gordon, J.A. & Sigurdsson, T. J. Neurosci. 33, 14211–14224 (2013).
Brincat, S.L. & Miller, E.K. Nat. Neurosci. 18, 576–581 (2015).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates 6th edn. (Academic Press, 2007).
Montaldo, G., Tanter, M., Bercoff, J., Benech, N. & Fink, M. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56, 489–506 (2009).
Bercoff, J. et al. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 134–147 (2011).
Acknowledgements
We gratefully acknowledge E. Yeramian and A. Bonnot for critical discussions and help with the manuscript. The research leading to these results has received funding from the European Research Council (ERC) under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 339244-FUSIMAGINE. This work was also partly supported by the Agence Nationale de la Recherche under the program “Future Investments” with the reference Laboratory of Excellence ANR-10-LABX-24 LABEX WIFI within the French Program “Investments for the Future” under reference ANR-10-IDEX-0001-02 PSL, the program “Investissements d'avenir” ANR-10-IAIHU-06 and a PhD fellowship from the University Paris Diderot FdV Bettencourt PhD program.
Author information
Authors and Affiliations
Contributions
L.-A.S. designed the surgical procedure and prosthetic skull and performed and analyzed epilepsy experiments; A.B. designed the intrahippocampal recording procedure and performed and analyzed navigation experiments; E.T. and T.D. developed the burst-mode ultrasound recording sequence; M.P. and J.-L.G. developed the continuous-mode ultrasound recording sequence; J.-L.G. and M.T. designed and supervised the ultrasound scanner and probe; I.C. designed and supervised the experiments, designed the probe holder, and programmed acquisition and analysis software; I.C., L.-A.S. and A.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Comparison of static and awake mobile fUS
The mfUS method is based on incremental improvements over functional ultrasound imaging on anesthetized rats.
(a, b) show side and front views of static fUS on anesthetized rat with open or thinned-bone craniotomy 5, 7. The rat is sedated and fixed in a stereotaxic frame.
(c) the PMP prosthesis surgical procedure and a first downsized ultrasound probe provide images in awake condition although the animal is highly constrained by the weight of the probe.
(d) a second generation, smaller and lighter, probe allows the animal to move and EEG to be recorded.
(e) comparison of second and third generation of probe show a further gain in size and weight.
(f) miniature motorized design allows to scan multiple planes without directly interacting with the animal, while the mechanical constraint is minimal. All the navigation and epilepsy measures presented here were performed with this smaller design.
Supplementary Figure 2 Surgical procedure
The surgical procedure to prepare the rat for simultaneous EEG and fUS recording proceeds in successive steps. First, a large bone flap is removed. Then electrodes are moved to the target position by stereotaxic micromotion, and they are anchored to the edge of the flap with a droplet of resin (a). For clarity, only one electrode is shown. Following sequential insertion of all the electrodes, the skull is replaced by an ultrasound clear prosthesis made of PMP. Finally, the prosthesis is sealed to the skull with resin and nuts are embedded in the resin for later attachment of the probe holder (b).
Supplementary Figure 3 Hippocampal electrodes
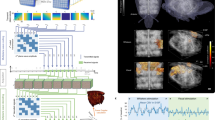
Hippocampal electrodes are made from a bundle of insulated tungsten wires soldered to a miniature connector at one end. Under binocular control the 50 µm reference electrode is juxtaposed with five 25 µm recording electrodes. The wires tips are positioned at fixed spacing in order to reach anatomical structures encompassing the dorsal hippocampus (a) and they are glued with cyanoacrylate in the upper part of the bundle to avoid accidentally covering the recording tips. A millimeter scale and microscope scale slide (total length 1 mm) are used to precisely set the spacing. Atlas from Paxinos19 (b). Differential recording from electrode tips 2-4, that show the typical theta band activation in relation with spatial navigation and phase reversal along the dorso-ventral axis are retained for analysis.
Supplementary Figure 4 Probe, holder and motor
Photographs of the miniature ultrasound probe (a), micromotor (b). The probe holder (c) is composed of a plate fixed onto the head (right) and a translation stage (left).
Supplementary Figure 5 CAD drawing of probe and holder parts
Front, side and top views of the base plate fixed to the head, mobile holder part and probe that fits into the mobile part. The base plate is attached to the head with M2 conical head screws. The probe and motor are secured with M2 headless screws. The sliding surface is lubricated with teflon spray. Digital information to reproduce the parts is provided as Supplementary Material 1.
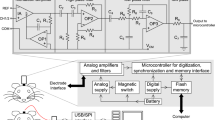
Supplementary Figure 6 Motor controller circuit
The motor controller circuit is based on an Arduino Uno open-source electronic platform. Requests for mechanical translation are received from the ultrasound scanner through a USB to serial converter based on a PL2303 chip. The Arduino turns on a DC-to-DC step down converter through the "ENABLE" pin to power the servomotor with optimal 3.7V voltage. Simultaneously the position information is sent by PWM modulation to the command line. Motor power is turned off. An LCD provides status information. Arduino microcode is provided in Supplementary Note.
Supplementary Figure 7 Comparison of "continuous" and "burst" modes
The continuous and burst modes make distinct usage of the ultrasound scanner acquisition and processing power. The main task is to transfer and process the large amount of high frequency plane wave scans into an fUltrasound images, which is saved as an array of single precision encoded pixel Power Doppler intensity corresponding to CBV. The reduction in data size is 1000 fold since 200 scans composed of repeats at 5 angles are used to generate one fUltrasound image.
The "continuous" mode has the advantage to permit acquisition of virtually unlimited duration. For this mode, the Ultrafast acquisition is set to form a single fUS image (200 ms). The transfer and processing time (here 2 seconds) limits the final rate of CBV maps.
The "burst" mode has the advantage to sustain the Ultrafast frame rate up to the filling of the RAM memories (here 12 s), allowing to capture short behavioral events such as maze crossing. CBV maps are reconstructed every 200 ms, but for this limited 12 s duration. Data is transferred in parallel with acquisition, and when buffer memory is full data is processed (40 s) to form a series of fUltrasound images. Thus duration of acquisition is limited by memory size, and interval duration is limited by processing speed.
The use of two modes is only due to technical limitations of current electronic boards of the ultrasound system. With improving bus technology and high end GPU boards it should be possible in theory today to build an Ultrafast ultrasound scanner with transfer and processing rates fast enough to work in the continuous mode during unlimited amount of time.
Supplementary Figure 8 Effect of recording procedure on running and seizures
(a, b) Maze experiment comparative distributions between mfUS-EEG (blue) and control untethered, surgery free, rats (red) show cumulative distance travelled in the maze is reduced by 36-44% while maximum speed is moderately reduced by 1-13%. Graphs show mean, median, center quartiles, 1 and 99 percentiles. (c, d) Genetic absence rats (GAERS, blue) show equivalent seizing time fraction and seizure duration distribution compared to EEG-only recordings (red). Graph c shows mean+/- s.d., 1 and 99 percentiles.
Detailed statistics are given in the Supplementary tables.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–3 and Supplementary Note (PDF 995 kb)
Supplementary Data
Probe Holder Manufacturing Template (PDF 301 kb)
Supplementary Software 1
Matlab code (ZIP 6 kb)
Supplementary Software 2
Labview VI code (ZIP 16558 kb)
Rights and permissions
About this article
Cite this article
Sieu, LA., Bergel, A., Tiran, E. et al. EEG and functional ultrasound imaging in mobile rats. Nat Methods 12, 831–834 (2015). https://doi.org/10.1038/nmeth.3506
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3506
This article is cited by
-
Decoding behavior from global cerebrovascular activity using neural networks
Scientific Reports (2023)
-
Global dissociation of the posterior amygdala from the rest of the brain during REM sleep
Communications Biology (2022)
-
Ultrasound localization microscopy and functional ultrasound imaging reveal atypical features of the trigeminal ganglion vasculature
Communications Biology (2022)
-
Whole-brain functional ultrasound imaging in awake head-fixed mice
Nature Protocols (2021)
-
Functional ultrasound imaging of the spreading activity following optogenetic stimulation of the rat visual cortex
Scientific Reports (2021)