Abstract
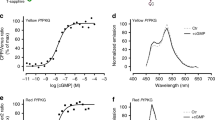
Fluorescence resonance energy transfer (FRET) from cyan to yellow fluorescent proteins (CFP/YFP) is a well-established method to monitor protein-protein interactions or conformational changes of individual proteins. But protein functions can be perturbed by fusion of large tags such as CFP and YFP. Here we use G protein–coupled receptor (GPCR) activation in living cells as a model system to compare YFP with the small, membrane-permeant fluorescein derivative with two arsen-(III) substituents (fluorescein arsenical hairpin binder; FlAsH) targeted to a short tetracysteine sequence. Insertion of CFP and YFP into human adenosine A2A receptors allowed us to use FRET to monitor receptor activation but eliminated coupling to adenylyl cyclase. The CFP/FlAsH-tetracysteine system gave fivefold greater agonist-induced FRET signals, similar kinetics (time constant of 66–88 ms) and perfectly normal downstream signaling. Similar results were obtained for the mouse α2A-adrenergic receptor. Thus, FRET from CFP to FlAsH reports GPCR activation in living cells without disturbing receptor function and shows that the small size of the tetracysteine-biarsenical tag can be decisively advantageous.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Miyawaki, A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev. Cell 4, 295–305 (2003).
Vilardaga, J.P., Bünemann, M., Krasel, C., Castro, M. & Lohse, M.J. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 21, 807–812 (2003).
Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 (2000).
Pierce, K.L., Premont, R.T. & Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 (2002).
Bissantz, C. Conformational changes of G protein-coupled receptors during their activation by agonist binding. J. Recept. Signal Transduct. Res. 23, 123–153 (2003).
Wess, J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 11, 346–354 (1997).
Greasley, P.J. et al. Mutational and computational analysis of the alpha(1b)-adrenergic receptor. Involvement of basic and hydrophobic residues in receptor activation and G protein coupling. J. Biol. Chem. 276, 46485–46494 (2001).
Milligan, G. Applications of bioluminescence- and fluorescence resonance energy transfer to drug discovery at G protein-coupled receptors. Eur. J. Pharm. Sci. 21, 397–405 (2004).
Griffin, B.A., Adams, S.R. & Tsien, R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 (1998).
Gaietta, G. et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 (2002).
Adams, S.R. et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 124, 6063–6076 (2002).
Ju, W. et al. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci. 7, 244–253 (2004).
Swaminathan, R., Hoang, C.P. & Verkman, A.S. Photobleaching recovery and anisotropy decay of green fluorescent protein GFP-S65T in solution and cells: cytoplasmic viscosity probed by green fluorescent protein translational and rotational diffusion. Biophys. J. 72, 1900–1907 (1997).
Swaminath, G. et al. Sequential binding of agonists to the β2 adrenoceptor. Kinetic evidence for intermediate conformational states. J. Biol. Chem. 279, 686–691 (2004).
Liapakis, G., Chan, W.C., Papadokostaki, M. & Javitch, J.A. Synergistic contributions of the functional groups of epinephrine to its affinity and efficacy at the beta2 adrenergic receptor. Mol. Pharmacol. 65, 1181–1190 (2004).
Cullen, B.R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 152, 684–704 (1987).
Bünemann, M., Frank, M. & Lohse, M.J. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. USA 100, 16077–16082 (2003).
Nikolaev, V.O., Bünemann, M., Hein, L., Hannawacker, A. & Lohse, M.J. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 279, 37215–37218 (2004).
Klotz, K.N. et al. Comparative pharmacology of human adenosine receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 357, 1–9 (1998).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (Leibniz award to M.J.L. and grant HO 2357/1-1 to C.H.), a Bavaria California Technology Center (BaCaTeC) grant to C.H., a Fonds der Chemischen Industrie grant to M.J.L. and US National Institutes of Health grant P41RR004050 to M.H.E.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The University of Würzburg has applied for a patent covering the technology described in this manuscript.
Supplementary information
Supplementary Video 1
FRET imaging of receptor activation in HEK-293 cell transiently transfected with α2A-AR-Flash/CFP. (MOV 1801 kb)
Rights and permissions
About this article
Cite this article
Hoffmann, C., Gaietta, G., Bünemann, M. et al. A FlAsH-based FRET approach to determine G protein–coupled receptor activation in living cells. Nat Methods 2, 171–176 (2005). https://doi.org/10.1038/nmeth742
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth742
This article is cited by
-
Function and dynamics of the intrinsically disordered carboxyl terminus of β2 adrenergic receptor
Nature Communications (2023)
-
A novel epitope tag from rabies virus has versatile in vitro applications
Applied Microbiology and Biotechnology (2023)
-
Determination of G-protein–coupled receptor oligomerization by molecular brightness analyses in single cells
Nature Protocols (2021)
-
Cartilage oligomeric matrix protein is an endogenous β-arrestin-2-selective allosteric modulator of AT1 receptor counteracting vascular injury
Cell Research (2021)
-
Structural insight into small molecule action on Frizzleds
Nature Communications (2020)