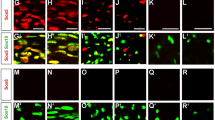
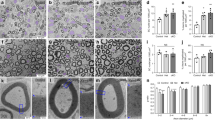
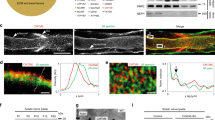
Abstract
Notch signaling is central to vertebrate development, and analysis of Notch has provided important insights into pathogenetic mechanisms in the CNS and many other tissues. However, surprisingly little is known about the role of Notch in the development and pathology of Schwann cells and peripheral nerves. Using transgenic mice and cell cultures, we found that Notch has complex and extensive regulatory functions in Schwann cells. Notch promoted the generation of Schwann cells from Schwann cell precursors and regulated the size of the Schwann cell pool by controlling proliferation. Notch inhibited myelination, establishing that myelination is subject to negative transcriptional regulation that opposes forward drives such as Krox20. Notably, in the adult, Notch dysregulation resulted in demyelination; this finding identifies a signaling pathway that induces myelin breakdown in vivo. These findings are relevant for understanding the molecular mechanisms that control Schwann cell plasticity and underlie nerve pathology, including demyelinating neuropathies and tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jessen, K.R. & Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6, 671–682 (2005).
Woodhoo, A. & Sommer, L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia 56, 1481–1490 (2008).
Chen, Z.L., Yu, W.M. & Strickland, S. Peripheral regeneration. Annu. Rev. Neurosci. 30, 209–233 (2007).
Louvi, A. & Artavanis-Tsakonas, S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 (2006).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 8, 709–715 (2005).
Lasky, J.L. & Wu, H. Notch signaling, brain development and human disease. Pediatr. Res. 57, 104R–109R (2005).
Bothwell, M. & Giniger, E. Alzheimer's disease: neurodevelopment converges with neurodegeneration. Cell 102, 271–273 (2000).
Jessen, K.R. & Mirsky, R. Negative regulation of myelination: relevance for development, injury and demyelinating disease. Glia 56, 1552–1565 (2008).
Scherer, S.S. & Salzer, J.L. Axonal-Schwann cell interactions during peripheral nerve degeneration and regeneration. in Glial Cell Development: Basic Principles and Clinical Relevance (eds. Jessen, K.R. & Richardson, W.D.) 299–330 (Oxford, New York, 2001).
Müller, H.W. & Stoll, G. Nerve injury and regeneration: basic insights and therapeutic interventions. Curr. Opin. Neurol. 11, 557–562 (1998).
Kubu, C.J. et al. Developmental changes in Notch1 and numb expression mediated by local cell-cell interactions underlie progressively increasing delta sensitivity in neural crest stem cells. Dev. Biol. 244, 199–214 (2002).
Wakamatsu, Y., Maynard, T.M. & Weston, J.A. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development 127, 2811–2821 (2000).
Morrison, S.J. et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499–510 (2000).
Bray, S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Taylor, M.K., Yeager, K. & Morrison, S.J. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development 134, 2435–2447 (2007).
Jessen, K.R., Morgan, L., Stewart, H.J.S. & Mirsky, R. Three markers of adult non-myelin-forming Schwann cells, 217c (Ran-1), A5E3 and GFAP: development and regulation by neuron–Schwann cell interactions. Development 109, 91–103 (1990).
Meier, C., Parmantier, E., Brennan, A., Mirsky, R. & Jessen, K.R. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3 and platelet-derived growth factor-BB. J. Neurosci. 19, 3847–3859 (1999).
Brennan, A. et al. Endothelins control the timing of Schwann cell generation in vitro and in vivo. Dev. Biol. 227, 545–557 (2000).
Dong, Z. et al. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation and maturation of rat Schwann cell precursors. Neuron 15, 585–596 (1995).
Birchmeier, C. & Nave, K.A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 (2008).
Stewart, H.J., Morgan, L., Jessen, K.R. & Mirsky, R. Changes in DNA synthesis rate in the Schwann cell lineage in vivo are correlated with the precursor-Schwann cell transition and myelination. Eur. J. Neurosci. 5, 1136–1144 (1993).
Yu, W.M., Feltri, M.L., Wrabetz, L., Strickland, S. & Chen, Z.L. Schwann cell–specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25, 4463–4472 (2005).
Winseck, A.K. & Oppenheim, R.W. An in vivo analysis of Schwann cell programmed cell death in embryonic mice: the role of axons, glial growth factor, and the pro-apoptotic gene Bax. Eur. J. Neurosci. 24, 2105–2117 (2006).
Webster, H.D., Martin, R. & O'Connell, M.F. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev. Biol. 32, 401–416 (1973).
Topilko, P. et al. Krox-20 controls myelination in the peripheral nervous system. Nature 371, 796–799 (1994).
Parkinson, D.B. et al. Regulation of the myelin gene periaxin provides evidence for Krox-20–independent myelin-related signaling in Schwann cells. Mol. Cell. Neurosci. 23, 13–27 (2003).
Parkinson, D.B. et al. c-Jun is a negative regulator of myelination. J. Cell Biol. 181, 625–637 (2008).
Lemke, G. & Chao, M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development 102, 499–504 (1988).
Morgan, L., Jessen, K.R. & Mirsky, R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP−, N-CAM−, NGF receptor−) depends on growth inhibition. J. Cell Biol. 112, 457–467 (1991).
Luo, D., Renault, V.M. & Rando, T.A. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 16, 612–622 (2005).
Martinez Arias, A., Zecchini, V. & Brennan, K. CSL-independent Notch signaling: a checkpoint in cell fate decisions during development. Curr. Opin. Genet. Dev. 12, 524–533 (2002).
Nave, K.A., Sereda, M.W. & Ehrenreich, H. Mechanisms of disease: inherited demyelinating neuropathies from basic to clinical research. Nat. Clin. Pract. Neurol. 3, 453–464 (2007).
Suter, U. & Scherer, S.S. Disease mechanisms in inherited neuropathies. Nat. Rev. Neurosci. 4, 714–726 (2003).
Svaren, J. & Meijer, D. The molecular machinery of myelin gene transcription in Schwann cells. Glia 56, 1541–1551 (2008).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Givogri, M.I. et al. Central nervous system myelination in mice with deficient expression of Notch1 receptor. J. Neurosci. Res. 67, 309–320 (2002).
John, G.R. et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat. Med. 8, 1115–1121 (2002).
Li, Y. et al. Notch and Schwann cell transformation. Oncogene 23, 1146–1152 (2004).
Hatakeyama, J. et al. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development 131, 5539–5550 (2004).
Tanigaki, K. et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat. Immunol. 3, 443–450 (2002).
Jaegle, M. et al. The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev. 17, 1380–1391 (2003).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Yang, X. et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev. Biol. 269, 81–94 (2004).
Feltri, M.L. et al. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann. NY Acad. Sci. 883, 116–123 (1999).
Sharghi-Namini, S. et al. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J. Neurosci. 26, 6364–6376 (2006).
D'Antonio, M. et al. TGFbeta type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J. Neurosci. 26, 8417–8427 (2006).
D'Antonio, M. et al. Gene profiling and bioinformatic analysis of Schwann cell embryonic development and myelination. Glia 53, 501–515 (2006).
Rangarajan, A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001).
Jessen, K.R., Morgan, L., Brammer, M. & Mirsky, R. Galactocerebroside is expressed by non-myelin-forming Schwann cells in situ. J. Cell Biol. 101, 1135–1143 (1985).
Morgan, L., Jessen, K.R. & Mirsky, R. Negative regulation of the P0 gene in Schwann cells: suppression of P0 mRNA and protein induction in cultured Schwann cells by FGF2 and TGF beta 1, TGF beta 2 and TGF beta 3. Development 120, 1399–1409 (1994).
Acknowledgements
This work was funded by a Wellcome Trust Programme Grant to K.R.J., R.M. and D.B.P., a Wellcome Trust Project grant to K.R.J. and R.M. and grants from the US National Institutes of Health to M.L.F. and L.W.
Author information
Authors and Affiliations
Contributions
A.W. carried out all the experiments with the exception of cAMP myelination assays and PCR analyses, which were performed by M.B.D.A. A.W. was helped by A.D. in in situ hybridization experiments, by M.T. in EM sectioning, by M.D. in FACS, by D.B.P. in in vitro inhibitor experiments and by D.K.W. in animal husbandry. R.A.-S. and P.S. generated Hes1−/−Hes5−/− cells from frozen embryos. J.S., F.G., F.R., D.M., M.L.F. and L.W. provided the mice. A.W. generated all the figures. A.W., R.M. and K.R.J. designed the experiments. K.R.J., A.W. and R.M. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–7 (PDF 5065 kb)
Rights and permissions
About this article
Cite this article
Woodhoo, A., Alonso, M., Droggiti, A. et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci 12, 839–847 (2009). https://doi.org/10.1038/nn.2323
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2323
This article is cited by
-
Hearing loss and vestibular schwannoma: new insights into Schwann cells implication
Cell Death & Disease (2023)
-
Molecular and Regenerative Characterization of Repair and Non-repair Schwann Cells
Cellular and Molecular Neurobiology (2023)
-
A topographic atlas defines developmental origins of cell heterogeneity in the human embryonic lung
Nature Cell Biology (2023)
-
Mature but not developing Schwann cells promote axon regeneration after peripheral nerve injury
npj Regenerative Medicine (2022)
-
The Notch signalling pathway and miRNA regulation play important roles in the differentiation of Schwann cells from adipose-derived stem cells
Laboratory Investigation (2022)