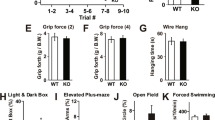
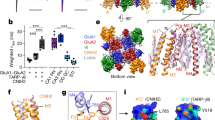
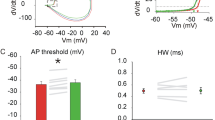
Abstract
Dynamic regulation of calcium-permeable AMPA receptors (CP-AMPARs) is important for normal synaptic transmission, plasticity and pathological changes. Although the involvement of transmembrane AMPAR regulatory proteins (TARPs) in trafficking of calcium-impermeable AMPARs (CI-AMPARs) has been extensively studied, their role in the surface expression and function of CP-AMPARs remains unclear. We examined AMPAR-mediated currents in cerebellar stellate cells from stargazer mice, which lack the prototypical TARP stargazin (γ-2). We found a marked increase in the contribution of CP-AMPARs to synaptic responses, indicating that, unlike CI-AMPARs, these can localize at synapses in the absence of γ-2. In contrast with CP-AMPARs in extrasynaptic regions, synaptic CP-AMPARs displayed an unexpectedly low channel conductance and strong block by intracellular spermine, suggesting that they were 'TARPless'. As a proof of principle that TARP association is not an absolute requirement for AMPAR clustering at synapses, miniature excitatory postsynaptic currents mediated by TARPless AMPARs were readily detected in stargazer granule cells following knockdown of their only other TARP, γ-7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Traynelis, S.F. et al. Glutamate receptor ion channels: structure, regulation and function. Pharmacol. Rev. 62, 405–496 (2010).
Chen, L. et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943 (2000).
Kato, A.S. et al. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J. Neurosci. 27, 4969–4977 (2007).
Soto, D. et al. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat. Neurosci. 12, 277–285 (2009).
Tomita, S. et al. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 161, 805–816 (2003).
Vandenberghe, W., Nicoll, R.A. & Bredt, D.S. Stargazin is an AMPA receptor auxiliary subunit. Proc. Natl. Acad. Sci. USA 102, 485–490 (2005).
Bats, C., Groc, L. & Choquet, D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734 (2007).
Schnell, E. et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. USA 99, 13902–13907 (2002).
Bedoukian, M.A., Weeks, A.M. & Partin, K.M. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J. Biol. Chem. 281, 23908–23921 (2006).
Cho, C.H., St-Gelais, F., Zhang, W., Tomita, S. & Howe, J.R. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron 55, 890–904 (2007).
Körber, C., Werner, M., Kott, S., Ma, Z.L. & Hollmann, M. The transmembrane AMPA receptor regulatory protein gamma 4 is a more effective modulator of AMPA receptor function than stargazin (gamma 2). J. Neurosci. 27, 8442–8447 (2007).
Milstein, A.D., Zhou, W., Karimzadegan, S., Bredt, D.S. & Nicoll, R.A. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron 55, 905–918 (2007).
Priel, A. et al. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J. Neurosci. 25, 2682–2686 (2005).
Soto, D., Coombs, I.D., Kelly, L., Farrant, M. & Cull-Candy, S.G. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat. Neurosci. 10, 1260–1267 (2007).
Tomita, S. et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435, 1052–1058 (2005).
Turetsky, D., Garringer, E. & Patneau, D.K. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J. Neurosci. 25, 7438–7448 (2005).
Kott, S., Sager, C., Tapken, D., Werner, M. & Hollmann, M. Comparative analysis of the pharmacology of GluR1 in complex with transmembrane AMPA receptor regulatory proteins gamma2, gamma3, gamma4, and gamma8. Neuroscience 158, 78–88 (2009).
Fukaya, M., Yamazaki, M., Sakimura, K. & Watanabe, M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci. Res. 53, 376–383 (2005).
Cull-Candy, S., Kelly, L. & Farrant, M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 16, 288–297 (2006).
Isaac, J.T., Ashby, M. & McBain, C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54, 859–871 (2007).
Liu, S.J. & Zukin, R.S. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 30, 126–134 (2007).
Bellone, C. & Luscher, C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 9, 636–641 (2006).
Chávez, A.E., Singer, J.H. & Diamond, J.S. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443, 705–708 (2006).
Gardner, S.M. et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron 45, 903–915 (2005).
Liu, S.Q.J. & Cull-Candy, S.G. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405, 454–458 (2000).
Rozov, A. & Burnashev, N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401, 594–598 (1999).
Kwak, S. & Weiss, J.H. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr. Opin. Neurobiol. 16, 281–287 (2006).
Hashimoto, K. et al. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J. Neurosci. 19, 6027–6036 (1999).
Yamazaki, M. et al. TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum. Eur. J. Neurosci. 31, 2204–2220 (2010).
Liu, Y. et al. A single fear-inducing stimulus induces a transcription-dependent switch in synaptic AMPAR phenotype. Nat. Neurosci. 13, 223–231 (2010).
Rossi, B., Maton, G. & Collin, T. Calcium-permeable presynaptic AMPA receptors in cerebellar molecular layer interneurones. J. Physiol. (Lond.) 586, 5129–5145 (2008).
Kelly, L., Farrant, M. & Cull-Candy, S.G. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat. Neurosci. 12, 593–601 (2009).
Liu, S.J. & Cull-Candy, S.G. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J. Neurosci. 22, 3881–3889 (2002).
Bowie, D., Lange, G.D. & Mayer, M.L. Activity-dependent modulation of glutamate receptors by polyamines. J. Neurosci. 18, 8175–8185 (1998).
Liu, S.J. & Cull-Candy, S.G. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat. Neurosci. 8, 768–775 (2005).
Fiszman, M.L. et al. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J. Neurosci. 25, 2024–2031 (2005).
Leitch, B., Shevtsova, O. & Kerr, J.R. Selective reduction in synaptic proteins involved in vesicle docking and signaling at synapses in the ataxic mutant mouse stargazer. J. Comp. Neurol. 512, 52–73 (2009).
Clark, B.A. & Cull-Candy, S.G. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor–only synapse. J. Neurosci. 22, 4428–4436 (2002).
Tóth, K. & McBain, C.J. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat. Neurosci. 1, 572–578 (1998).
Feldmeyer, D. et al. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat. Neurosci. 2, 57–64 (1999).
Swanson, G.T., Kamboj, S.K. & Cull-Candy, S.G. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation and subunit composition. J. Neurosci. 17, 58–69 (1997).
Tardin, C., Cognet, L., Bats, C., Lounis, B. & Choquet, D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 22, 4656–4665 (2003).
Sun, L. & June Liu, S. Activation of extrasynaptic NMDA receptors induces a PKC-dependent switch in AMPA receptor subtypes in mouse cerebellar stellate cells. J. Physiol. (Lond.) 583, 537–553 (2007).
Menuz, K., Stroud, R.M., Nicoll, R.A. & Hays, F.A. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science 318, 815–817 (2007).
Menuz, K., O'Brien, J.L., Karmizadegan, S., Bredt, D.S. & Nicoll, R.A. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. 28, 8740–8746 (2008).
Cathala, L., Holderith, N.B., Nusser, Z., DiGregorio, D.A. & Cull-Candy, S.G. Changes in synaptic structure underlie the developmental speeding of AMPA receptor–mediated EPSCs. Nat. Neurosci. 8, 1310–1318 (2005).
Meng, H., Walker, N., Su, Y. & Qiao, X. Stargazin mutation impairs cerebellar synaptogenesis, synaptic maturation and synaptic protein distribution. Brain Res. 1124, 197–207 (2006).
Richardson, C.A. & Leitch, B. Phenotype of cerebellar glutamatergic neurons is altered in stargazer mutant mice lacking brain-derived neurotrophic factor mRNA expression. J. Comp. Neurol. 481, 145–159 (2005).
Gill, M.B. et al. Cornichon-2 modulates AMPA receptor–transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology. J. Neurosci. 31, 6928–6938 (2011).
Jackson, A.C. & Nicoll, R.A. Stargazin (TARP gamma-2) is required for compartment-specific AMPA receptor trafficking and synaptic plasticity in cerebellar stellate cells. J. Neurosci. 31, 3939–3952 (2011).
López-Bendito, G. et al. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb. Cortex 14, 1122–1133 (2004).
Letts, V.A. et al. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat. Genet. 19, 340–347 (1998).
Kudoh, S.N. & Taguchi, T. A simple exploratory algorithm for the accurate and fast detection of spontaneous synaptic events. Biosens. Bioelectron. 17, 773–782 (2002).
Chung, C., Deak, F. & Kavalali, E.T. Molecular substrates mediating lanthanide-evoked neurotransmitter release in central synapses. J. Neurophysiol. 100, 2089–2100 (2008).
Traynelis, S.F., Silver, R.A. & Cull-Candy, S.G. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber–granule cell synapse. Neuron 11, 279–289 (1993).
Acknowledgements
We thank all members of the S.G.C.-C./M.F. laboratory for invaluable discussions. We are grateful to L. Kelly and A.V. Stempel for help during the early stages of the project and to I. Coombs for assistance with molecular biology. M. Yamasaki and M. Watanabe (Hokkaido University Graduate School of Medicine) generously shared unpublished data with us. We thank G. Szabó (Institute of Experimental Medicine) for GAD65-eGFP mice, kindly provided by M. Häusser (Wolfson Institute, University College London), and M. Hastings (Medical Research Council, Laboratory of Molecular Biology) for stargazer mice. This work was supported by a Wellcome Trust Programme Grant (S.G.C.-C. and M.F.) and a Medical Research Council Programme Grant (S.G.C.-C. and M.F.). C.B. was supported by a Marie Curie Intra-European Fellowship.
Author information
Authors and Affiliations
Contributions
Experiments were performed by C.B. (stellate cell recordings), D. Studniarczyk (granule cell recordings) and D. Soto (tsA201 cell and stellate cell recordings). M.F., C.B., D. Soto and D. Studniarczyk analyzed the data. S.G.C.-C. and M.F. supervised the project. All of the authors contributed to the design and interpretation of experiments. C.B., M.F. and S.G.C.-C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1, 2 and Supplementary Tables 1–3 (PDF 205 kb)
Rights and permissions
About this article
Cite this article
Bats, C., Soto, D., Studniarczyk, D. et al. Channel properties reveal differential expression of TARPed and TARPless AMPARs in stargazer neurons. Nat Neurosci 15, 853–861 (2012). https://doi.org/10.1038/nn.3107
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3107
This article is cited by
-
AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking
Cellular and Molecular Life Sciences (2019)
-
Synaptic AMPA receptor composition in development, plasticity and disease
Nature Reviews Neuroscience (2016)
-
Modulation of AMPA receptor function by auxiliary subunits
e-Neuroforum (2015)
-
Inhibition of AMPA Receptors by Polyamine Toxins is Regulated by Agonist Efficacy and Stargazin
Neurochemical Research (2014)
-
TARP γ-7 selectively enhances synaptic expression of calcium-permeable AMPARs
Nature Neuroscience (2013)