Abstract
The ability to regulate the consolidation and strengthening of memories for threatening experiences is critical for mental health, and its dysregulation may lead to psychopathologies. Re-exposure to the context in which the threat was experienced can either increase or decrease fear response through distinct processes known, respectively, as reconsolidation or extinction. Using a context retrieval-dependent memory-enhancement model in rats, we report that memory strengthens through activation of direct projections from dorsal hippocampus to prelimbic (PL) cortex and activation of critical PL molecular mechanisms that are not required for extinction. Furthermore, while sustained PL brain-derived neurotrophic factor (BDNF) expression is required for memory consolidation, retrieval engages PL BDNF to regulate excitatory and inhibitory synaptic proteins neuroligin 1 and neuroligin 2, which promote memory strengthening while inhibiting extinction. Thus, context retrieval-mediated fear-memory enhancement results from a concerted action of mechanisms that strengthen memory through reconsolidation while suppressing extinction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 (2004).
McGaugh, J.L. Memory--a century of consolidation. Science 287, 248–251 (2000).
Squire, L.R., Stark, C.E. & Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004).
Frankland, P.W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005).
Kim, J.J. & Fanselow, M.S. Modality-specific retrograde amnesia of fear. Science 256, 675–677 (1992).
Nadel, L. & Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 7, 217–227 (1997).
Inda, M.C., Muravieva, E.V. & Alberini, C.M. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 31, 1635–1643 (2011).
Fukushima, H. et al. Enhancement of fear memory by retrieval through reconsolidation. eLife 3, e02736 (2014).
Berman, D.E. & Dudai, Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291, 2417–2419 (2001).
Santini, E., Ge, H., Ren, K., Peña de Ortiz, S. & Quirk, G.J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 24, 5704–5710 (2004).
Eisenberg, M., Kobilo, T., Berman, D.E. & Dudai, Y. Stability of retrieved memory: inverse correlation with trace dominance. Science 301, 1102–1104 (2003).
Suzuki, A. et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795 (2004).
Milekic, M.H., Pollonini, G. & Alberini, C.M. Temporal requirement of C/EBPbeta in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learn. Mem. 14, 504–511 (2007).
Alberini, C.M. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 28, 51–56 (2005).
Korb, E. & Finkbeiner, S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 34, 591–598 (2011).
Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 89, 121–145 (2009).
Gu, J. et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat. Neurosci. 13, 1208–1215 (2010).
Schwindel, C.D. & McNaughton, B.L. Hippocampal-cortical interactions and the dynamics of memory trace reactivation. Prog. Brain Res. 193, 163–177 (2011).
Sierra-Mercado, D., Padilla-Coreano, N. & Quirk, G.J. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36, 529–538 (2011).
Sotres-Bayon, F. & Quirk, G.J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 20, 231–235 (2010).
Urban, D.J. & Roth, B.L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
Stachniak, T.J., Ghosh, A. & Sternson, S.M. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014).
Armbruster, B.N., Li, X., Pausch, M.H., Herlitze, S. & Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 104, 5163–5168 (2007).
Hoover, W.B. & Vertes, R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 212, 149–179 (2007).
Xu, W. & Südhof, T.C. A neural circuit for memory specificity and generalization. Science 339, 1290–1295 (2013).
DeNardo, L.A., Berns, D.S., DeLoach, K. & Luo, L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat. Neurosci. 18, 1687–1697 (2015).
Bambah-Mukku, D., Travaglia, A., Chen, D.Y., Pollonini, G. & Alberini, C.M. A positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J. Neurosci. 34, 12547–12559 (2014).
Rex, C.S. et al. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J. Neurosci. 27, 3017–3029 (2007).
Gottmann, K., Mittmann, T. & Lessmann, V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp. Brain Res. 199, 203–234 (2009).
Riga, D. et al. Optogenetic dissection of medial prefrontal cortex circuitry. Front. Syst. Neurosci. 8, 230 (2014).
Courtin, J. et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Choi, D.C., Gourley, S.L. & Ressler, K.J. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl. Psychiatry 2, e205 (2012).
Choi, D.C. et al. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc. Natl. Acad. Sci. USA 107, 2675–2680 (2010).
Liang, J. et al. Conditional neuroligin-2 knockout in adult medial prefrontal cortex links chronic changes in synaptic inhibition to cognitive impairments. Mol. Psychiatry 20, 850–859 (2015).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Rosas-Vidal, L.E., Do-Monte, F.H., Sotres-Bayon, F. & Quirk, G.J. Hippocampal--prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology 39, 2161–2169 (2014).
Miller, E.K. & Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001).
Baldi, E. & Bucherelli, C. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci. Biobehav. Rev. 53, 160–190 (2015).
Marquis, J.P., Killcross, S. & Haddon, J.E. Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur. J. Neurosci. 25, 559–566 (2007).
Ragozzino, M.E. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann. NY Acad. Sci. 1121, 355–375 (2007).
Acknowledgements
We thank W.-J. Lin, N. Humala, G. Pollonini, K. Pandey, L. Barboza and the personnel of the NYU animal facilities for technical support. We thank G. Philips and W.-J. Lin for feedback on the manuscript. This work was supported by grant R01-MH074736 to C.M.A. and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to X.Y.
Author information
Authors and Affiliations
Contributions
X.Y., D.K.-L., A.T., M.C.I. and C.M.A. designed and developed this study. X.Y., D.K.-L., A.T. and M.C.I. carried out the experiments. X.Y., D.K.-L., A.T. and C.M.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
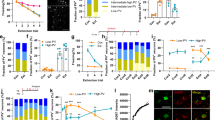
Supplementary Figure 1 The behavioral outcomes of blocking Arc expression in dHC and BLA during retrieval.
(a) Mean latency ± s.e.m. of rats injected (black arrow) with Arc AS or SC into dHC 2 d after training (Tr) and memory retention tested (T) 1 h later (unpaired two-tailed Student t-test; t14 = 0.2768, P = 0.786, n = 8, 8; 2 independent experiments). (b) Mean latency ± s.e.m. of rats injected (black arrows) bilaterally into the BLA with Arc antisense (AS) or control scrambled (SC) oligodeoxynucleotides (ODNs) 1 h before each 10 s retrieval in the 3 retrievals (3Rs) group or at the matched time points after training (Tr) in the non-retrieval (NoR) group (two-way ANOVA followed by Bonferroni post hoc test; treatment F3, 65 = 50.82, P < 0.0001; testing F2, 65 = 2.01, P = 0.1418; interaction F6, 65 = 0.98, P = 0.4470; n = 8, 8, 9, 8; 3 independent experiments). T1-T3: tests as shown in the schema. RS: reminder footshock. * P < 0.05, ** P < 0.01, *** P < 0.001. Histological images showing the injection sites are presented in Supplementary Figure 10.
Supplementary Figure 2 Context retrieval does not change the levels of total CREB, cofilin or TrkB in dHC and mPFC.
Examples and relative quantitative western blots analyses of dHC and mPFC extracts obtained from rats trained (Tr) and euthanized (eut, red arrows) 1 h after 1 or 3 memory retrievals (1R or 3Rs), or 3 exposures to a novel context (3Cs), or at the matched time point in the non-retrieval (NoR) group. Naïve rats (N) served as reference control. Data are presented as mean percentage ± s.e.m. of the mean values of the N group (one-way ANOVA followed by Newman-Keuls post hoc test; dHC: CREB F4, 30 = 0.3876, P = 0.8158, n = 9, 7, 5, 8, 6; cofilin F4, 30 = 0.2894, P = 0.8825, n = 9, 7, 5, 8, 6; mPFC: CREB F4, 32 = 0.6654, P = 0.6206, n = 9, 8, 5, 8, 7; cofilin F4, 32 = 1.545, P = 0.2128, n = 9, 8, 5, 8, 7; TrkB F4, 32 = 0.5108, P = 0.7282, n = 9, 8, 5, 8, 7; 3 independent experiments).
Supplementary Figure 3 Both memory enhancement and extinction increase the levels of Arc, pCREB and p-cofilin in dHC.
Cropped examples and relative quantitative western blots analyses of dHC extracts obtained from rats trained (Tr) and euthanized (eut, red arrows) 1 h after 3 memory retrievals (3Rs) or extinction (T + Ext), or at the matched time point in the non-retrieval (NoR) group. Naïve rats (N) served as reference control. Data are presented as mean percentage ± s.e.m. of the mean values of the N group (one-way ANOVA followed by Newman-Keuls post hoc test; Arc F3, 25 = 10.03, P = 0.0002, n = 8, 7, 7, 7; pCREB F3, 24 = 9.310, P = 0.0003, n = 7, 7, 7, 7; pcofilin F3, 25 = 7.325, P = 0.0011, n = 8, 7, 7, 7; 3 independent experiments). * P < 0.05, ** P < 0.01, *** P < 0.001. Full-length blots are presented in Supplementary Figure 11.
Supplementary Figure 4 Immunofluorescent staining of brain sections following dHC injection of AAV8/hSyn-HA-hM4Di-IRES-mCitrine.
Staining for HA-hM4Di is shown in red, mCitrine in green, the nuclear marker DAPI in blue, and merged images are shown on the right. Images of corresponding brain atlas are shown on the right of the merged immunofluorescent staining images, with red box indicating where the immunofluorescent staining images are taken from. (a) Images taken with Olympus VS120 wide-field epi-fluorescent microscope showing immunofluorescent staining of mCitrine and HA-hM4Di in dHC. (b) Images taken with Leica SP5 confocal microscope showing immunofluorescent staining of mCitrine and HA-hM4Di in the PL and IL. The images on the right show enlarged views of dHC projection terminals in the PL and IL cortex that contain hM4Di, corresponding to the white boxed - area in the merged images.
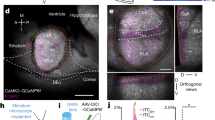
Supplementary Figure 5 Retrograde labeling of dHC neurons projecting to PL; and Arc expression in mPFC after blocking dHC-to-PL cortex projections.
(a) Distribution of the retrograde tracer CTB-Alexa Fluor 555 at the injection site in PL, as well as the spread and retrograde labeling of neurons in adjacent mPFC sections. (b) Retrograde labeling of hippocampal neurons in the same brain injected with CTB-Alexa Fluor 555 in PL. Note that there is sparse labeling of dorsal hippocampal neurons in CA1 and CA2 subregions, indicating direct projections from this part of hippocampus to PL. The images were taken with Olympus VS120 wide-field epi-fluorescent microscope: CTB-Alexa Fluor 555 (red), DAPI (blue). Enlarged images on the bottom are taken from the white boxed - area in the upper images. (c) Examples and quantification of immunofluorescent staining of Arc in the PL and IL obtained from rats euthanized (eut, red arrows) 1 h after 3 memory retrievals (3Rs) or at the matched time points for the non-retrieval (NoR) groups from the rats injected with AAV8/hSyn-HA-hM4Di-IRES-mCitrine or control AAV8/hSyn-GFP into dHC, and CNO or control vehicle (veh) into PL (black arrows). Data are presented as mean percentage ± s.e.m. of the mean values of the NoR + veh group (one-way ANOVA followed by Newman-Keuls post hoc test; PL F4, 21 = 25.36, P < 0.0001, n = 5, 5, 6, 5, 5; IL F4, 21 = 8.694, P = 0.0003, n = 5, 5, 6, 5, 5; 2 independent experiments). ** P < 0.01, *** P < 0.001.
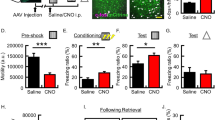
Supplementary Figure 6 Chicago Sky Blue dye diffusion in PL; and memory retention after blocking Arc expression in PL.
(a) Representative sections of one rat brain at +3.24mm, +2.76mm and +2.52mm from bregma, analyzed under light microscopy for Chicago Sky Blue (1%) diffusion 1 h after PL injection (6 independent experiments). (b) Mean latency ± s.e.m. of rats bilaterally injected (black arrows) into the PL cortex with Arc antisense (AS) or control scrambled (SC) oligodeoxynucleotides (ODNs) 1 h before each 10 s retrieval in the 3 retrievals (3Rs) group or at the matched time points after training (Tr) in the non-retrieval (NoR) group. Memory retention was tested (T1-T3) as shown in the schema. RS: reminder footshock (two-way ANOVA followed by Bonferroni post hoc test; treatment F2, 88 = 60.88, P < 0.0001; testing F2, 88 = 0.57, P = 0.5688; interaction F4, 88 = 0.73, P = 0.5714; n = 12, 13, 13; 3 independent experiments). ** P < 0.01, *** P < 0.001. Histological images showing the injection sites are presented in Supplementary Figure 10.
Supplementary Figure 7 Training and context retrieval-mediated induction of GluA1 and GluA2.
Cropped examples and relative quantitative western blots analyses of mPFC extracts obtained from rats trained (Tr) and euthanized (eut, red arrows) 1 h after 1 or 3 memory retrievals (1R or 3Rs), or 3 exposures to a novel context (3Cs), or at the matched time point in the non-retrieval (NoR) group. Naïve rats (N) served as reference control. Data are presented as mean percentage ± s.e.m. of the mean values of the N group (one-way ANOVA followed by Newman-Keuls post hoc test; GluA1 F4, 26 = 6.146, P = 0.0013, n = 10, 8, 4, 5, 4; GluA2 F4, 26 = 4.557, P = 0.0064, n = 10, 8, 4, 5, 4; 3 independent experiments). * P < 0.05, ** P < 0.01, *** P < 0.001. Full-length blots are presented in Supplementary Figure 12.
Supplementary Figure 8 Molecular changes in the PL cortex after training, memory enhancement and extinction.
Cropped examples and relative quantitative western blot analyses of PL extracts obtained from rats trained (Tr) and euthanized (eut, red arrows) 1 h after 3 memory retrievals (3Rs), extinction (T + Ext) or at the matched time point in the non-retrieval (NoR) group. Naïve rats (N) served as reference control. Data are presented as mean percentage ± s.e.m. of the mean values of the N group (one-way ANOVA followed by Newman-Keuls post hoc test; Arc F3, 33 = 15.82, P < 0.0001, n = 10, 9, 9, 9; pCREB F3, 21 = 8.344, P = 0.0008, n = 6, 6, 6, 7; pcofilin F3, 22 = 7.033, P = 0.0017, n = 6, 6, 7, 7; BDNF F3, 22 = 3.421, P = 0.0350, n = 6, 6, 7, 7; pTrkB F3, 21 = 5.461, P = 0.0062, n = 6, 6, 7, 7; NLGN1 F3, 33 = 4.449, P = 0.0099, n = 10, 9, 9, 9; NLGN2 F3, 34 = 3.620, P = 0.0227, n = 10, 9, 10, 9; 3 independent experiments). Full-length blots are presented in Supplementary Figure 12.
Supplementary Figure 9 Training, retrieval and extinction do not change actin level.
Cropped examples and relative quantitative western blots analyses of PL extracts obtained from rats trained (Tr) and euthanized (eut, red arrows) 1 h after 3 memory retrievals (3Rs) or extinction (T + Ext), or at the matched time point in the non-retrieval (NoR) group. Naïve rats (N) served as reference control. Data are presented as mean percentage ± s.e.m. of the mean values of the N group (one-way ANOVA followed by Newman-Keuls post hoc test; actin per lane F3, 28 = 0.2485, P = 0.8617, n = 8, 8, 8, 8; actin / GAPDH F3, 28 = 0.3735, P = 0.7727, n = 8, 8, 8, 8; 3 independent experiments).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 2557 kb)
Rights and permissions
About this article
Cite this article
Ye, X., Kapeller-Libermann, D., Travaglia, A. et al. Direct dorsal hippocampal–prelimbic cortex connections strengthen fear memories. Nat Neurosci 20, 52–61 (2017). https://doi.org/10.1038/nn.4443
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4443
This article is cited by
-
Dorsal hippocampus to nucleus accumbens projections drive reinforcement via activation of accumbal dynorphin neurons
Nature Communications (2024)
-
Differential recruitment of brain circuits during fear extinction in non-stressed compared to stress resilient animals
Scientific Reports (2024)
-
Plexin-A1 expression in the inhibitory neurons of infralimbic cortex regulates the specificity of fear memory in male mice
Neuropsychopharmacology (2022)
-
Molecular motor KIF3B in the prelimbic cortex constrains the consolidation of contextual fear memory
Molecular Brain (2021)
-
Microglial deletion and inhibition alleviate behavior of post-traumatic stress disorder in mice
Journal of Neuroinflammation (2021)