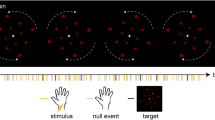
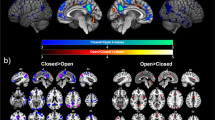
Abstract
To investigate the neural substrates underlying emotional feelings in the absence of a conscious stimulus percept, we presented a visual stimulus in the blind field of partially cortically blind patients and measured cortical activity (by functional magnetic resonance imaging, fMRI) before and after the stimulus had been paired with an aversive event. After pairing, self-reported negative emotional valence and blood oxygen level–dependent (BOLD) responses in somatosensory association areas were enhanced, whereby somatosensory activity predicted highly corresponding reported feelings and startle reflex amplitudes across subjects. Our data provide direct evidence that cortical activity representing physical emotional states governs emotional feelings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weiskrantz, L. Brain 126, 265–266 (2003).
LeDoux, J.E. Curr. Opin. Neurobiol. 2, 191 (1992).
Linke, R., De Lima, A.D., Schwegler, H. & Pape, H.C. J. Comp. Neurol. 403, 158–170 (1999).
Morris, J.S., DeGelder, B., Weiskrantz, L. & Dolan, R.J. Brain 124, 1241–1252 (2001).
Hamm, A.O. et al. Brain 126, 267–275 (2003).
Adolphs, R., Damasio, H., Tranel, D., Cooper, G. & Damasio, A.R. J. Neurosci. 20, 2683–2690 (2000).
Damasio, A.R. et al. Nat. Neurosci. 3, 1049–1056 (2000).
Kimmig, H., Greenlee, M.W., Huethe, F. & Mergner, T. Exp. Brain Res. 126, 443–449 (1999).
Friston, K.J. et al. Hum. Brain Mapp. 2, 189–210 (1995).
Price, C.J. & Friston, K.J. Neuroimage 5, 261–270 (1997).
Collins, D.L., Neelin, P., Peters, T.M. & Evans, A.C. J. Comput. Assist. Tomogr. 18, 192–205 (1994).
Bradley, M.M. & Lang, P.J. J. Behav. Ther. Exp. Psychiatry 25, 49–59 (1994).
Rosen, J.B. et al. J. Neurosci. 12, 4624–4633 (1992).
Hitchcock, J.M. & Davis, M. Behav. Neurosci. 105, 826–842 (1991).
Pissiota, A., Frans, O., Fredrikson, M., Langstrom, B. & Flaten, M.A. Eur. J. Neurosci. 15, 395–398 (2002).
Acknowledgements
We thank H. Flor, K. Mathiak, R. Veit, N. Weiskopf, L. Weiskrantz and D. Wildgruber for helpful discussions, B. Newport, M. Hülsmann and B. Wietek for technical support, and H.O. Karnath, P. Stoerig and U. Schiefer for permitting us to include patients from their wards. This study was partly supported by the Volkswagen Foundation and the Junior Science Program of the Heidelberger Academy of Sciences and Humanities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Anatomical sections (patients 1-4 with infarction of the primary visual cortex) or fractional diffusion anisotropy maps (patients 5-9 with lesions affecting the optic radiation). Arrows indicate infarction or lesion. Left hemisphere appears at left side of image (neurological convention). (JPG 62 kb)
Supplementary Table 1
Clinical data and visual field defects, assessed at a Tübinger Perimeter, of each patient. Hand, dominant hand; (r) retrained right hander; Time, time since lesion in years. Patient numbers refer to those shown in Supplementary Fig. 1. (PDF 48 kb)
Rights and permissions
About this article
Cite this article
Anders, S., Birbaumer, N., Sadowski, B. et al. Parietal somatosensory association cortex mediates affective blindsight. Nat Neurosci 7, 339–340 (2004). https://doi.org/10.1038/nn1213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1213
This article is cited by
-
Viewpoints: Approaches to defining and investigating fear
Nature Neuroscience (2019)
-
Visual and noxious electrical stimulus-evoked membrane-potential responses in anterior cingulate cortical neurons
Molecular Brain (2016)
-
Neural bases of the non-conscious perception of emotional signals
Nature Reviews Neuroscience (2010)
-
Attention and amygdala activity: an fMRI study with spider pictures in spider phobia
Journal of Neural Transmission (2009)
-
Toward and away from spiders: eye-movements in spider-fearful participants
Journal of Neural Transmission (2009)