Abstract
Hypothalamic pro-opiomelanocortin (POMC) neurons help regulate long-term energy stores. POMC neurons are also found in the nucleus tractus solitarius (NTS), a region regulating satiety. We show here that mouse brainstem NTS POMC neurons are activated by cholecystokinin (CCK) and feeding-induced satiety and that activation of the neuronal melanocortin-4 receptor (MC4-R) is required for CCK-induced suppression of feeding; the melanocortin system thus provides a potential substrate for integration of long-term adipostatic and short-term satiety signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fan, W., Boston, B.A., Kesterson, R.A., Hruby, V.J. & Cone, R.D. Nature 385, 165–168 (1997).
Cheung, C.C., Clifton, D.K. & Steiner, R.A. Endocrinol. 138, 4489–4492 (1997).
Elias, C.F. et al. Neuron 23, 775–786 (1999).
Cowley, M.A. et al. Nature 411, 480–484 (2001).
Williams, D.L., Grill, H.J., Weiss, S.M., Baird, J.P. & Kaplan, J.M. Psychopharmacology 161, 47–53 (2002).
Azzara, A.V., Sokolnicki, J.P. & Schwartz, G.J. Physiol. Behav. 77, 411–416 (2002).
Grill, H.J., Ginsberg, A.B., Seeley, R.J. & Kaplan, J.M. J. Neurosci. 18, 10128–10135 (1998).
Joseph, S.A., Pilcher, W.H. & Bennet-Clarke, C. Neurosci. Lett. 38, 221–225 (1983).
Wang, L., Martinez, V., Barrachina, M.D. & Tache, Y. Brain Res. 791, 157–166 (1998).
Rinaman, L. Am. J. Physiol. 277, R582–R590 (1997).
Luckman, S. J. Neuroendocrinol. 4, 149–152 (1992).
Butler, A.A. et al. Endocrinol. 141, 3518–3521 (2000).
Huszar, D. et al. Cell 88, 131–141 (1997).
Hruby, V.J. et al. J. Med. Chem. 38, 3454–3461 (1995).
Farooqi, I.S. et al. N. Engl. J. Med. 348, 1160–1163 (2003).
Acknowledgements
Supported by US National Institutes of Health grants DK55819 (R.D.C.) and DK62179 (W.F.), and a grant from the Wellcome Trust (K.L.J.E.). POMC-EGFP mice were a kind gift of M. Low (Oregon Health and Science University).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.D.C. and Oregon Health and Science University have a significant financial interest in Neurocrine Biosciences, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committee.
Supplementary information
Supplementary Fig. 1
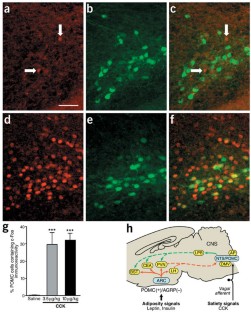
Feeding activates NTS POMC neurons. (a) c-Fos-IR neurons in the NTS 60 minutes after food intake (b) POMC-EGFP-IR cells in the NTS 60 minutes after food intake. (c) Colocalization of c-Fos and POMC in NTS neurons 60 minutes after food intake. (d) The percent of NTS POMC neurons at 1100h expressing c-Fos in ARC and NTS in control (fasted) animals, or animals receiving food (fed) at 9000-1000h. (e) Percent of c-Fos-IR neurons in ARC or NTS expressing POMC-EGFP in animals in the fed (n=8) or fasted state (n=3). ***P<0.001. Size bar (a) = 100μM. Arrows indicate cells expressing both EGFP-IR and c-Fos-IR. (JPG 38 kb)
Supplementary Fig. 2
POMC expression defines a unique population of TH-negative, GLP-1-negative cells within the NTS. (a) Anti-tyrosine hydroxylase (TH) antibodies identify distinct TH-positive neurons (red) within in the same region of the NTS as POMC-EGFP-IR neurons. (b) Anti-EGFP antibodies are used to detect POMC neurons (green) in the NTS of the EGFP-POMC mouse. (c) No coexpression of POMC and TH expression was observed in individual neurons of the mouse NTS. (d,e) POMC-EGFP-IR cells were generally found medial of GLP-1-IR cells in the NTS. No co-expression of the peptides was observed. Size bars indicate 135 μM (a-c), 250 μM (d), and 50 μM (e). CC, central canal; AP, area postrema. (JPG 42 kb)
Rights and permissions
About this article
Cite this article
Fan, W., Ellacott, K., Halatchev, I. et al. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7, 335–336 (2004). https://doi.org/10.1038/nn1214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1214
This article is cited by
-
Gut microbiota and type 2 diabetes mellitus: a focus on the gut-brain axis
Endocrine (2024)
-
The biological functions and metabolic pathways of valine in swine
Journal of Animal Science and Biotechnology (2023)
-
SK3 in POMC neurons plays a sexually dimorphic role in energy and glucose homeostasis
Cell & Bioscience (2022)
-
Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding
Nature Metabolism (2022)
-
Autonomic control of energy balance and glucose homeostasis
Experimental & Molecular Medicine (2022)