Abstract
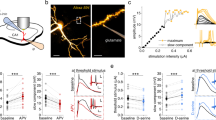
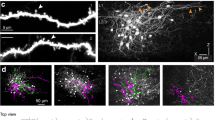
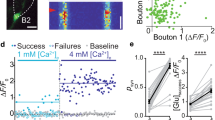
Here we demonstrate that cerebellar stellate cells diffusionally isolate synaptically evoked signals in dendrites and are capable of input-specific synaptic plasticity. Sustained activity of parallel fibers induces a form of long-term depression that requires opening of calcium (Ca2+)-permeable AMPA-type glutamate receptors (CP-AMPARs) and signaling through class 1 metabotropic glutamate receptors (mGluR1) and CB1 receptors. This depression is induced by postsynaptic increases in Ca2+ concentration ([Ca2+]) and is limited to activated synapses. To understand how synapse-specific plasticity is induced by diffusible second messengers in aspiny dendrites, we examined diffusion of Ca2+ and small molecules within stellate cell dendrites. Activation of a single parallel fiber opened CP-AMPARs, generating long-lived Ca2+ transients that were confined to submicron dendritic stretches. The diffusion of Ca2+ was severely retarded due to interactions with parvalbumin and a general restriction of small molecule mobility. Thus stellate cell dendrites spatially restrict signaling cascades that lead from CP-AMPAR activation to endocannabinoid production and trigger the selective regulation of active synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Nimchinsky, E.A., Sabatini, B.L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002).
Palay, S.L. & Chan-Palay, V. The Stellate Cell, 216–233 (Strange-Verlag, New York, 1974).
Freund, T.F. & Buzsaki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
McMahon, L.L. & Kauer, J.A. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron 18, 295–305 (1997).
Cowan, A.I., Stricker, C., Reece, L.J. & Redman, S.J. Long-term plasticity at excitatory synapses on aspinous interneurons in area CA1 lacks synaptic specificity. J. Neurophysiol. 79, 13–20 (1998).
Rozsa, B., Zelles, T., Vizi, E.S. & Lendvai, B. Distance-dependent scaling of calcium transients evoked by backpropagating spikes and synaptic activity in dendrites of hippocampal interneurons. J. Neurosci. 24, 661–670 (2004).
Kaiser, K.M., Lubke, J., Zilberter, Y. & Sakmann, B. Postsynaptic calcium influx at single synaptic contacts between pyramidal neurons and bitufted interneurons in layer 2/3 of rat neocortex is enhanced by backpropagating action potentials. J. Neurosci. 24, 1319–1329 (2004).
Topolnik, L., Congar, P. & Lacaille, J.C. Differential regulation of metabotropic glutamate receptor- and AMPA receptor-mediated dendritic Ca2+ signals by presynaptic and postsynaptic activity in hippocampal interneurons. J. Neurosci. 25, 990–1001 (2005).
Goldberg, J.H., Tamas, G., Aronov, D. & Yuste, R. Calcium microdomains in aspiny dendrites. Neuron 40, 807–821 (2003).
Hausser, M. & Clark, B.A. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19, 665–678 (1997).
Liu, S.Q. & Cull-Candy, S.G. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405, 454–458 (2000).
Rancillac, A. & Crepel, F. Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J. Physiol. (Lond.) 554, 707–720 (2004).
Kosaka, T., Kosaka, K., Nakayama, T., Hunziker, W. & Heizmann, C.W. Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Exp. Brain Res. 93, 483–491 (1993).
Bowie, D. & Mayer, M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 (1995).
Kamboj, S.K., Swanson, G.T. & Cull-Candy, S.G. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J. Physiol. (Lond.) 486, 297–303 (1995).
Koh, D.S., Burnashev, N. & Jonas, P. Block of native Ca(2+)-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J. Physiol. (Lond.) 486, 305–312 (1995).
Carter, A.G. & Regehr, W.G. Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J. Neurosci. 20, 4423–4434 (2000).
Washburn, M.S. & Dingledine, R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J. Pharmacol. Exp. Ther. 278, 669–678 (1996).
Herkenham, M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann. NY Acad. Sci. 654, 19–32 (1992).
Kreitzer, A.C. & Regehr, W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29, 717–727 (2001).
Maejima, T., Ohno-Shosaku, T. & Kano, M. Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci. Res. 40, 205–210 (2001).
Rancillac, A. & Barbara, J.G. Frequency-dependent recruitment of inhibition mediated by stellate cells in the rat cerebellar cortex. J. Neurosci. Res. 80, 414–423 (2005).
Brown, S.P., Brenowitz, S.D. & Regehr, W.G. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat. Neurosci. 6, 1048–1057 (2003).
Brenowitz, S.D. & Regehr, W.G. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J. Neurosci. 23, 6373–6384 (2003).
Safo, P.K. & Regehr, W.G. Endocannabinoids control the induction of cerebellar LTD. Neuron 48, 647–659 (2005).
Sabatini, B.L., Oertner, T.G. & Svoboda, K. The life cycle of Ca2+ ions in dendritic spines. Neuron 33, 439–452 (2002).
Carter, A.G. & Regehr, W.G. Quantal events shape cerebellar interneuron firing. Nat. Neurosci. 5, 1309–1318 (2002).
Zador, A. & Koch, C. Linearized models of calcium dynamics: formal equivalence to the cable equation. J. Neurosci. 14, 4705–4715 (1994).
Gabso, M., Neher, E. & Spira, M.E. Low mobility of the Ca2+ buffers in axons of cultured Aplysia neurons. Neuron 18, 473–481 (1997).
Toth, K. & McBain, C.J. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat. Neurosci. 1, 572–578 (1998).
Neher, E. & Augustine, G.J. Calcium gradients and buffers in bovine chromaffin cells. J. Physiol. (Lond.) 450, 273–301 (1992).
Zhou, Z. & Neher, E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J. Physiol. (Lond.) 469, 245–273 (1993).
Kushmerick, M.J. & Podolsky, R.J. Ionic mobility in muscle cells. Science 166, 1297–1298 (1969).
Michailova, A., DelPrincipe, F., Egger, M. & Niggli, E. Spatiotemporal features of Ca2+ buffering and diffusion in atrial cardiac myocytes with inhibited sarcoplasmic reticulum. Biophys. J. 83, 3134–3151 (2002).
Hou, T.T., Johnson, J.D. & Rall, J.A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J. Physiol. (Lond.) 441, 285–304 (1991).
Lee, S.H., Schwaller, B. & Neher, E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: implications for [Ca2+] transients of neuronal dendrites. J. Physiol. (Lond.) 525, 419–432 (2000).
Li-Smerin, Y., Levitan, E.S. & Johnson, J.W. Free intracellular Mg2+ concentration and inhibition of NMDA responses in cultured rat neurons. J. Physiol. (Lond.) 533, 729–743 (2001).
Carter, A.G. & Sabatini, B.L. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44, 483–493 (2004).
Bloodgood, B.L. & Sabatini, B.L. Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310, 866–869 (2005).
Jasuja, R., Keyoung, J., Reid, G.P., Trentham, D.R. & Khan, S. Chemotactic responses of Escherichia coli to small jumps of photoreleased L-aspartate. Biophys. J. 76, 1706–1719 (1999).
Xia, P., Bungay, P.M., Gibson, C.C., Kovbasnjuk, O.N. & Spring, K.R. Diffusion coefficients in the lateral intercellular spaces of Madin-Darby canine kidney cell epithelium determined with caged compounds. Biophys. J. 74, 3302–3312 (1998).
Svoboda, K., Tank, D.W. & Denk, W. Direct measurement of coupling between dendritic spines and shafts. Science 272, 716–719 (1996).
Levenes, C., Daniel, H., Soubrie, P. & Crepel, F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J. Physiol. (Lond.) 510, 867–879 (1998).
Maejima, T. et al. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J. Neurosci. 25, 6826–6835 (2005).
Allbritton, N.L., Meyer, T. & Stryer, L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258, 1812–1815 (1992).
Strautman, A.F., Cork, R.J. & Robinson, K.R. The distribution of free calcium in transected spinal axons and its modulation by applied electrical fields. J. Neurosci. 10, 3564–3575 (1990).
Popov, S. & Poo, M.M. Diffusional transport of macromolecules in developing nerve processes. J. Neurosci. 12, 77–85 (1992).
Ellis, R.J. Macromolecular crowding: obvious but underappreciated. Trends Biochem. Sci. 26, 597–604 (2001).
Yasuda, R. et al. Imaging calcium concentration dynamics in small neuronal compartments. Sci. STKE 2004, pl5 (2004).
Pologruto, T.A., Sabatini, B.L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Acknowledgements
We thank members of the Sabatini lab and W. Regehr for helpful discussions, and J. Corrie and D. Ogden for the gift of NPE-HPTS. This work was funded by the National Institutes of Neurological Disorders and Stroke (1 F31 NS049655-01) and Quan predoctoral fellowships to G.J.S.L., and a Burroughs Wellcome Fund Career Award, a Searle Scholar award, and McKnight Foundation Technological Innovations Awards to B.L.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Time course of an experiment showing the increase in failure rate after the induction of SCLTD. (PDF 191 kb)
Supplementary Fig. 2
Cyclopyazonic acid (CPA) abolishes caffeine-induced Ca2+ transients in cerebellar Purkinje cells. (PDF 3354 kb)
Supplementary Fig. 3
Summary of EPSC amplitudes in the tetanized and control pathways in the presence of the class 1 mGluR antagonist CPCCOEt (50 μM) (n = 7). (PDF 200 kb)
Supplementary Fig. 4
ΔG/Rsyn plotted against EPSC amplitude for 528 trials from the cells used in Figure 4 (n = 13 cells). (PDF 329 kb)
Supplementary Fig. 5
Analytical model of Ca2+ diffusion and clearance in SC dendrites. (PDF 616 kb)
Rights and permissions
About this article
Cite this article
Soler-Llavina, G., Sabatini, B. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci 9, 798–806 (2006). https://doi.org/10.1038/nn1698
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1698
This article is cited by
-
Afferent convergence to a shared population of interneuron AMPA receptors
Nature Communications (2023)
-
Opposing actions of CRF-R1 and CB1 receptor on facial stimulation-induced MLI-PC plasticity in mouse cerebellar cortex
BMC Neuroscience (2022)
-
A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo
Nature Biotechnology (2022)
-
Gating by Functionally Indivisible Cerebellar Circuits: a Hypothesis
The Cerebellum (2021)
-
Gating by Memory: a Theory of Learning in the Cerebellum
The Cerebellum (2021)