Abstract
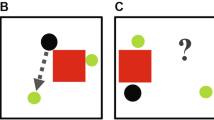
The ability to interpret and predict other people's actions is highly evolved in humans and is believed to play a central role in their cognitive behavior. However, there is no direct evidence that this ability confers a tangible benefit to sensory processing. Our quantitative behavioral experiments show that visual discrimination of a human agent is influenced by the presence of a second agent. This effect depended on whether the two agents interacted (by fighting or dancing) in a meaningful synchronized fashion that allowed the actions of one agent to serve as predictors for the expected actions of the other agent, even though synchronization was irrelevant to the visual discrimination task. Our results demonstrate that action understanding has a pervasive impact on the human ability to extract visual information from the actions of other humans, providing quantitative evidence of its significance for sensory performance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Puce, A. & Perrett, D. Electrophysiology and brain imaging of biological motion. Phil. Trans. R. Soc. Lond. B 358, 435–445 (2003).
Nelissen, K., Vanduffel, W. & Orban, G.A. Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. J. Neurosci. 26, 5929–5947 (2006).
Rizzolatti, G. & Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 (2004).
Gallese, V., Fadiga, L., Fogassi, L. & Rizzolatti, G. Action representation and the inferior parietal lobule. in Common Mechanisms in Perception and Action: Attention and Performance XIX (eds. Prinz, W. & Hommel, B.) 247–266 (Oxford Univ. Press, Oxford, 2002).
Giese, M.A. & Poggio, T. Neural mechanisms for the recognition of biological motion. Nat. Rev. Neurosci. 4, 179–192 (2003).
Bülthoff, I., Bülthoff, H. & Sinha, P. Top-down influences on stereoscopic depth-perception. Nat. Neurosci. 1, 254–257 (1998).
Neri, P., Morrone, M.C. & Burr, D.C. Seeing biological motion. Nature 395, 894–896 (1998).
Blake, R., Turner, L.M., Smoski, M.J., Pozdol, S.L. & Stone, W.L. Visual recognition of biological motion is impaired in children with autism. Psychol. Sci. 14, 151–157 (2003).
Barlow, H.B. Cerebral cortex as model builder. in Models of the Visual Cortex (eds. Rose, D. & Dobson, V.G.) 37–46 (John Wiley & Sons, New York, 1985).
Lange, J. & Lappe, M. A model of biological motion perception from configural form cues. J. Neurosci. 26, 2894–2906 (2006).
Morrone, M.C., Burr, D.C. & Vaina, L.M. Two stages of visual processing for radial and circular motion. Nature 376, 507–509 (1995).
Sumi, S. Upside down presentation of the Johannson moving light spot pattern. Perception 13, 283–286 (1984).
Pavlova, M. & Sokolov, A. Orientation specificity in biological motion perception. Percept. Psychophys. 62, 889–899 (2000).
Brown, W.M. et al. Dance reveals symmetry especially in young men. Nature 438, 1148–1150 (2005).
Oram, M. & Perrett, D.I. Responses of anterior superior temporal polysensory (STPa) neurons to 'biological motion' stimuli. J. Cogn. Neurosci. 6, 99–116 (1994).
Bonda, E., Petrides, M., Ostry, D. & Evans, A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J. Neurosci. 16, 3737–3744 (1996).
Grossman, E.D. & Blake, R. Brain areas active during visual perception of biological motion. Neuron 35, 1167–1175 (2002).
Castelli, F., Happé, F., Frith, U. & Frith, C. Movement and mind: a functional study of perception and interpretation of complex intentional movement patterns. Neuroimage 12, 314–325 (2000).
Martin, A. & Weisberg, J. Neural foundations for understanding social and mechanical concepts. Cogn. Neuropsychol. 20, 575–587 (2003).
Schultz, J., Friston, K.J., O'Doherty, J., Wolpert, D.M. & Frith, C.D. Activation in posterior superior temporal sulcus parallels parameter inducing the percept of animacy. Neuron 45, 625–635 (2005).
Gallese, V. & Goldman, A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci. 2, 493–501 (1998).
Kohler, E. et al. Hearing sounds, understanding actions: action representation in mirror neurons. Science 297, 846–848 (2002).
Umiltà, M.A. et al. I know what you are doing: a neurophysiological study. Neuron 31, 155–165 (2001).
Nelissen, K., Luppino, G., Vanduffel, W., Rizzolatti, G. & Orban, G.A. Observing others: multiple action representation in the frontal lobe. Science 310, 332–336 (2005).
Rizzolatti, G., Fogassi, L. & Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2, 661–670 (2001).
Fadiga, L., Fogassi, L., Pavesi, G. & Rizzolatti, G. Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol. 73, 2608–2611 (1995).
Iacoboni, M. et al. Cortical mechanisms of human imitation. Science 286, 2526–2528 (1999).
Peelen, M.V., Wiggett, A.J. & Downing, P.E. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron 49, 815–822 (2006).
Nishitani, N. & Hari, R. Temporal dynamics of cortical representation for action. Proc. Natl. Acad. Sci. USA 97, 913–918 (2000).
Iacoboni, M. et al. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 3, E79 (2005).
Calvo-Merino, B., Glaser, D.E., Grzes, J., Passingham, R.E. & Haggard, P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb. Cortex 15, 1243–1249 (2005).
Frith, C.D. & Frith, U. The neural basis of mentalizing. Neuron 50, 531–534 (2006).
Adolphs, R. Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 4, 165–178 (2003).
Gallese, V., Keysers, C. & Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 8, 396–403 (2004).
Williams, J.H., Whiten, A., Suddendorf, T. & Perrett, D.I. Imitation, mirror neurons and autism. Neurosci. Biobehav. Rev. 25, 287–295 (2001).
Oberman, L.M. et al. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Res. Cogn. Brain. Res. 24, 190–198 (2005).
Dapretto, M. et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 9, 28–30 (2006).
Dittrich, W.H., Troscianko, T., Lea, S. & Morgan, D. Perception of emotion from dynamic point-light displays represented in dance. Perception 25, 727–738 (1996).
Bassili, J.N. Facial motion in the perception of faces and of emotional expression. J. Exp. Psychol. Hum. Percept. Perform. 4, 373–379 (1978).
Barraclough, N.E., Xiao, D., Baker, C.I., Oram, M.W. & Perrett, D.I. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J. Cogn. Neurosci. 17, 377–391 (2005).
Keysers, C. & Perrett, D.I. Demystifying social cognition: a Hebbian perspective. Trends Cogn. Sci. 8, 501–507 (2004).
Iacoboni, M. et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proc. Natl. Acad. Sci. USA 98, 13995–13999 (2001).
Kersten, D., Mamassian, P. & Yuille, A. Object perception as Bayesian inference. Annu. Rev. Psychol. 55, 271–304 (2004).
Burr, D. Motion smear. Nature 284, 164–165 (1980).
Acknowledgements
Supported by US National Institutes of Health grant RO1EY01728.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Motion-captured fighting and dancing sequences. Top panels show original movies of actors wearing light markers while performing a fighting (left) and a dancing (right) sequence. Bottom panels show corresponding motion-captured sequences after tracking of the joints. (MPG 2976 kb)
Supplementary Video 2
Comparison between Sync and de-Sync stimuli. The four panels containing point-light agents show an animated version of Fig. 2d–g. Black arrows schematically depict the operation of computing inter-dot distance for all possible dot pairs on each frame. Black traces in the upper-right graph plot the average magnitude of this distance for the Sync sequences, grey for the de-Sync sequences. Yellow arrows show the average motion vector for all dots (computed between position at frame t and frame t+1). Dark yellow traces in the upper-right graph plot the magnitude of this vector for Sync sequences, lighter yellow for de-Sync. Green double-arrows point from the average dot position of one agent to the average dot position for the other agent. Dark green traces in upper-right graph plot the magnitude of this inter-agent distance for de-Sync sequences, lighter green for Sync. The graph at the bottom-right plots distributions for the quantities shown in the upper graph, updated as the sequences progress over time. These distributions are indistinguishable at P > 0.05 for first and second-order statistics. We also ran programs that attempted to distinguish between Sync and de-Sync using extracts from these sequences that were equal in length to the ones we used in our experiments, but failed to differentiate between the two. We also measured these statistics for the inverted control sequences and found them to be virtually identical to those in the original non-inverted sequence. All sequences shown here are for fighters, but we obtained similar results with dancers. (MPG 2498 kb)
Supplementary Video 3
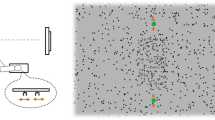
Examples of limited-lifetime sampling, noise and agent inversion. This demo consists of 6 iterations, 3 for the sequences used in the main experiment and 3 for the sequences containing one inverted agent that were used in the control experiment. All sequences shown here are for fighters. Left panels show two examples of 'target' and 'non-target' intervals for Sync trials, right panels for de-Sync. Three modes of presentation are demonstrated (labelled in red at bottom): unlimited dot lifetime, limited dot lifetime, and limited dot lifetime with added noise dots. Each iteration lasts more than 3 seconds for ease of inspection, but we used a presentation time of 1.5 seconds for fighters in the actual experiments. To ease comparison across the different panels, similar extracts have been used in all of them. However, the trajectories extracted from the original movie for display in the psychophysical experiments were randomly selected independently for every interval on every trial. Based on the characteristics of the sequences we used and on the presentation modes that are demonstrated in this movie, we can list at least five reasons why we think inter-agent occlusion could not be the source of the differential Sync/de-Sync effect we report in Fig. 2. First, because dots have a limited lifetime in our stimuli (see also Fig. 1i–j) they effectively disappear and reappear all the time, thus disappearance by occlusion is not special (see second iteration in this movie). Second, in the presence of noise dots, occlusion rules are constantly violated by the noise dots themselves, which can appear anywhere within and outside the agents (see third iteration in this movie). Third, the inverted control experiment preserves occlusion almost entirely, because both agents are still present and occlude each other in almost identical ways, despite the fact that one of them is upside-down (see second set of iterations in this movie). Fourth, the percentage of occlusion induced by one agent on the other agent (inter-agent occlusion) is only a fraction of the occlusion induced by one agent on himself/herself (self-occlusion): 46% for the fighting sample, 23% for the dancing sample. Self-occlusion is unrelated to synchronization between agents. If occlusion plays any role, it would be dominated by self-occlusion, leaving little scope for inter-agent occlusion to generate the differential effect reported in Fig. 2. Finally, the psychophysical effect was stronger for dancing than for fighting (compare blue and red symbols in Fig. 2a). If it were due to inter-agent occlusion, we would expect that such occlusion would be more pronounced in the dancing sequence than in the fighting sequence. To the contrary, inter-agent occlusion is more pronounced in the fighting sequence (8% of total sequence as opposed to 6% for dancers). The unlimited dot lifetime experiment (open black symbols in Fig. 2b and first iteration in this Video) serves as a further control for a potential role of inter-agent occlusion, because such occlusion is enhanced in fully sampled sequences. If inter-agent occlusion were the source of the effect, we would expect it to be even stronger in the fully sampled condition (the opposite of what we observe). Finally, this experiment adds evidence to the notion that the reduced performance on de-Sync trials cannot be attributed to observers being 'put off' by desynchronization and refusing to perform the task altogether (criterion change). Not only were they not aware of desynchronization during testing, but they could perform extremely well on de-Sync trials when the joint trajectories are fully sampled, a manipulation that enhances the perceptual impact of synchronization (mean percent correct for detecting synchronization was 90% in the fully sampled condition, as opposed to 77% in the undersampled version; paired t-test across observers for former greater than latter returns P < 0.05). (MPG 1964 kb)
Supplementary Video 4
Procedure used to measure noise and scrambling thresholds. For noise thresholds (top), as the number of noise dots progressively increases, performance drops from 100% correct (1) to chance (0.5) in the graph at top right. Yellow dots show real data for one subject (from the inset in Fig. 2a), dot size scales with number of trials. Blue line shows weighted (by square root of number of trials) Weibull fit. The number of noise dots is gradually increased in this demonstration, but in the actual experiments it was only varied from one trial to the next and never within a presentation. For scrambling thresholds (bottom), this demonstration shows three iterations of a motion sequence where one of the two agents in the non-target stimulus is progressively less scrambled. As scrambling decreases, the ability to distinguish target from non-target interval drops from 100% correct to chance. Yellow dots show real data from one of the insets in Fig. 2b. (MPG 2009 kb)
Rights and permissions
About this article
Cite this article
Neri, P., Luu, J. & Levi, D. Meaningful interactions can enhance visual discrimination of human agents. Nat Neurosci 9, 1186–1192 (2006). https://doi.org/10.1038/nn1759
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1759
This article is cited by
-
Forms of prediction in the nervous system
Nature Reviews Neuroscience (2020)
-
A social interaction field model accurately identifies static and dynamic social groupings
Nature Human Behaviour (2019)
-
Automatic attribution of social coordination information to chasing scenes: evidence from mu suppression
Experimental Brain Research (2018)
-
EEG frequency tagging dissociates between neural processing of motion synchrony and human quality of multiple point-light dancers
Scientific Reports (2017)
-
Observing human movements helps decoding environmental forces
Experimental Brain Research (2011)