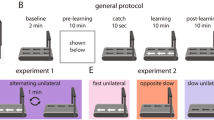
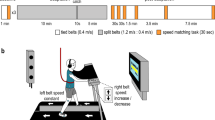
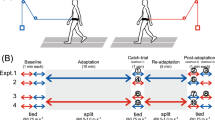
Abstract
Human walking is remarkably adaptable on short and long timescales. We can immediately transition between directions and gait patterns, and we can adaptively learn accurate calibrations for different walking contexts. Here we studied the degree to which different motor patterns can adapt independently. We used a split-belt treadmill to adapt the right and left legs to different speeds and in different directions (forward versus backward). To our surprise, adults could easily walk with their legs moving in opposite directions. Analysis of aftereffects showed that walking adaptations are stored independently for each leg and do not transfer across directions. Thus, there are separate functional networks controlling forward and backward walking in humans, and the circuits controlling the right and left legs can be trained individually. Such training could provide a new therapeutic approach for correcting various walking asymmetries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lam, T., Anderschitz, M. & Dietz, V. Contribution of feedback and feedforward strategies to locomotor adaptations. J. Neurophysiol. 95, 766–773 (2006).
Pearson, K.G. Neural adaptation in the generation of rhythmic behavior. Annu. Rev. Physiol. 62, 723–753 (2000).
Reisman, D.S., Block, H.J. & Bastian, A.J. Interlimb coordination during locomotion: what can be adapted and stored? J. Neurophysiol. 94, 2403–2415 (2005).
Morton, S.M. & Bastian, A.J. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J. Neurosci. 26, 9107–9116 (2006).
Reisman, D.S., Wityk, R., Silver, K. & Bastian, A.J. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain, published online 2 April 2007 (doi:10.1093/brain/awm035).
Grillner, S. & Zangger, P. On the central generation of locomotion in the low spinal cat. Exp. Brain Res. 34, 241–261 (1979).
Mortin, L.I. & Stein, P.S. Spinal cord segments containing key elements of the central pattern generators for three forms of scratch reflex in the turtle. J. Neurosci. 9, 2285–2296 (1989).
Calancie, B. et al. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 117, 1143–1159 (1994).
Dimitrijevic, M.R., Gerasimenko, Y. & Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. NY Acad. Sci. 860, 360–376 (1998).
Grasso, R., Bianchi, L. & Lacquaniti, F. Motor patterns for human gait: backward versus forward locomotion. J. Neurophysiol. 80, 1868–1885 (1998).
Lafreniere-Roula, M. & McCrea, D.A. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J. Neurophysiol. 94, 1120–1132 (2005).
Rybak, I.A., Shevtsova, N.A., Lafreniere-Roula, M. & McCrea, D.A. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J. Physiol. (Lond.) 577, 617–639 (2006).
Lamb, T. & Yang, J.F. Could different directions of infant stepping be controlled by the same locomotor central pattern generator? J. Neurophysiol. 83, 2814–2824 (2000).
Yang, J.F., Lamont, E.V. & Pang, M.Y. Split-belt treadmill stepping in infants suggests autonomous pattern generators for the left and right leg in humans. J. Neurosci. 25, 6869–6876 (2005).
Dietz, V., Zijlstra, W. & Duysens, J. Human neuronal interlimb coordination during split-belt locomotion. Exp. Brain Res. 101, 513–520 (1994).
Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306 (2006).
Hammar, I., Bannatyne, B.A., Maxwell, D.J., Edgley, S.A. & Jankowska, E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur. J. Neurosci. 19, 1305–1316 (2004).
Morton, S.M. & Bastian, A.J. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J. Neurophysiol. 92, 2497–2509 (2004).
Krakauer, J.W., Mazzoni, P., Ghazizadeh, A., Ravindran, R. & Shadmehr, R. Generalization of motor learning depends on the history of prior action. PLoS Biol. 4, e316 (2006).
Reynolds, R.F. & Bronstein, A.M. The moving platform aftereffect: limited generalization of a locomotor adaptation. J. Neurophysiol. 91, 92–100 (2004).
Yanagihara, D. & Kondo, I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc. Natl. Acad. Sci. USA 93, 13292–13297 (1996).
Horak, F.B. & Diener, H.C. Cerebellar control of postural scaling and central set in stance. J. Neurophysiol. 72, 479–493 (1994).
Lang, C.E. & Bastian, A.J. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J. Neurophysiol. 82, 2108–2119 (1999).
Smith, M.A. & Shadmehr, R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J. Neurophysiol. 93, 2809–2821 (2005).
Bastian, A.J. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr. Opin. Neurobiol. 16, 645–649 (2006).
Bosco, G., Poppele, R.E. & Eian, J. Reference frames for spinal proprioception: limb-endpoint based or joint-level based? J. Neurophysiol. 83, 2931–2945 (2000).
Bosco, G., Eian, J. & Poppele, R.E. Kinematic and nonkinematic signals transmitted to the cat cerebellum during passive treadmill stepping. Exp. Brain Res. 167, 394–403 (2005).
Bosco, G., Eian, J. & Poppele, R.E. Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp. Brain Res. 175, 83–96 (2006).
Poppele, R.E., Rankin, A. & Eian, J. Dorsal spinocerebellar tract neurons respond to contralateral limb stepping. Exp. Brain Res. 149, 361–370 (2003).
Arshavsky, Y.I., Gelfand, I.M., Orlovsky, G.N. & Pavlova, G.A. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res. 151, 493–506 (1978).
Orlovsky, G.N. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res. 40, 359–371 (1972).
Orlovsky, G.N. Activity of vestibulospinal neurons during locomotion. Brain Res. 46, 85–98 (1972).
Yang, J.F. et al. Infant stepping: a window to the behaviour of the human pattern generator for walking. Can. J. Physiol. Pharmacol. 82, 662–674 (2004).
Forssberg, H., Grillner, S., Halbertsma, J. & Rossignol, S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol. Scand. 108, 283–295 (1980).
Berkowitz, A. & Stein, P.S. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: phase analyses. J. Neurosci. 14, 5105–5119 (1994).
Kullander, K. et al. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299, 1889–1892 (2003).
Batschelet, E. Circular Statistics in Biology (Academic Press, New York, 1981).
Zar, J.H. Biostatistical Analysis (Prentice Hall, Upper Saddle River, New Jersey, 1999).
Acknowledgements
Thanks to D. Reisman, S. Morton, J. Bastian and E. Connor for thoughtful discussions and input about these studies, and to A. Torrie for help with testing. This work was supported by US National Institutes of Health R01HD048740 and C06RR15488.
Author information
Authors and Affiliations
Contributions
J.T.C. and A.J.B. designed the experiments and cowrote the manuscript. J.T.C. conducted the experiments and data analyses.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Animation of experiment I: test of transfer from forward to backward walking. (MOV 1208 kb)
Supplementary Video 2
Animation of experiment II: test of transfer from backward to forward walking. (MOV 1344 kb)
Supplementary Video 3
Animation of experiment III: test of dual adaptation in backward and forward walking. (MOV 1919 kb)
Supplementary Video 4
Animation of hybrid adaptation: hybrid-walking adaptation. (MOV 819 kb)
Supplementary Video 5
Animation of experiment IV: test of transfer from hybrid walking to forward and backward walking. (MOV 1843 kb)
Rights and permissions
About this article
Cite this article
Choi, J., Bastian, A. Adaptation reveals independent control networks for human walking. Nat Neurosci 10, 1055–1062 (2007). https://doi.org/10.1038/nn1930
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1930
This article is cited by
-
Can a passive unilateral hip exosuit diminish walking asymmetry? A randomized trial
Journal of NeuroEngineering and Rehabilitation (2023)
-
Different functional networks underlying human walking with pulling force fields acting in forward or backward directions
Scientific Reports (2023)
-
A mental workload and biomechanical assessment during split-belt locomotor adaptation with and without optic flow
Experimental Brain Research (2023)
-
Stepping in Decerebrated Cats at Simultaneously Different Speeds on a Split Treadmill
Neuroscience and Behavioral Physiology (2023)
-
Gender differences in the effect of a 0.11% breath alcohol concentration on forward and backward gait
Scientific Reports (2022)