Abstract
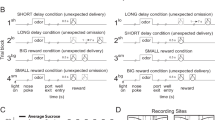
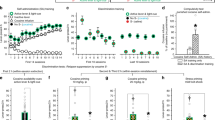
Addicts and drug-experienced animals have decision-making deficits in reversal-learning tasks and more complex 'gambling' variants. Here we show evidence that these deficits are mediated by persistent encoding of outdated associative information in the basolateral amygdala. Cue-selective neurons in the basolateral amygdala, recorded in cocaine-treated rats, failed to change cue preference during reversal learning. Further, the presence of these neurons was critical to the expression of the reversal-learning deficit in the cocaine-treated rats.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schoenbaum, G., Saddoris, M.P., Ramus, S.J., Shaham, Y. & Setlow, B. Eur. J. Neurosci. 19, 1997–2002 (2004).
Jentsch, J.D., Olausson, P., De La Garza, R. & Taylor, J.R. Neuropsychopharmacology 26, 183–190 (2002).
Calu, D.J. et al. Learn. Mem. 14, 325–328 (2007).
Bechara, A. et al. Neuropsychologia 39, 376–389 (2001).
Stalnaker, T.A., Franz, T.M., Singh, T. & Schoenbaum, G. Neuron 54, 51–58 (2007).
Saddoris, M.P., Gallagher, M. & Schoenbaum, G. Neuron 46, 321–331 (2005).
Schoenbaum, G., Chiba, A.A. & Gallagher, M. J. Neurosci. 19, 1876–1884 (1999).
Paton, J.J., Belova, M.A., Morrison, S.E. & Salzman, C.D. Nature 439, 865–870 (2006).
Schoenbaum, G., Setlow, B., Nugent, S.L., Saddoris, M.P. & Gallagher, M. Learn. Mem. 10, 129–140 (2003).
Izquierdo, A. & Murray, E.A. J. Neurosci. 27, 1054–1062 (2007).
Carelli, R.M., Williams, J.G. & Hollander, J.A. J. Neurosci. 23, 8204–8211 (2003).
Fuchs, R.A., Feltenstein, M.W. & See, R.E. Eur. J. Neurosci. 23, 2809–2813 (2006).
See, R.E., McLaughlin, J. & Fuchs, R.A. Neuroscience 117, 477–483 (2003).
Lee, J.L., Di Ciano, P., Thomas, K.L. & Everitt, B.J. Neuron 47, 795–801 (2005).
Milad, M.R. & Quirk, G.J. Nature 420, 70–74 (2002).
Acknowledgements
We are grateful to S. Warrenburg at International Flavors and Fragrances for his assistance in obtaining odor compounds. This work was supported by grants from the National Institute on Drug Abuse (DA015718, G.S.), the National Institute of Neurological Diseases and Stroke (T32-NS07375, M.R.R.) and the National Institute on Deafness and other Communication Disorders (T32-DC00054, T.S.).
Author information
Authors and Affiliations
Contributions
T.A.S. and G.S. conceived the experiments; T.A.S., M.R.R. and D.J.C. carried out the recording work; T.A.S. and T.S. carried out the lesion work; and T.M.F. assisted with electrode construction, surgeries and histology. The data were analyzed by T.A.S. and G.S., who also cowrote the manuscript with assistance from each of the other team members.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Effect of cocaine on locomotor activity. (PDF 46 kb)
Supplementary Fig. 2
Average baseline firing rate and distribution of firing rates for neurons recorded in saline- and cocaine-treated rats. (PDF 15 kb)
Supplementary Fig. 3
Behavioral performance in saline- and cocaine-treated rats during recording sessions. (PDF 26 kb)
Supplementary Fig. 4
Effect of cocaine treatment on the percentage of cue-selective ABL neurons that normally reverse cue-preference during reversal learning. (PDF 34 kb)
Supplementary Fig. 5
Effect of cocaine and ABL lesions on locomotor activity. (PDF 49 kb)
Rights and permissions
About this article
Cite this article
Stalnaker, T., Roesch, M., Franz, T. et al. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nat Neurosci 10, 949–951 (2007). https://doi.org/10.1038/nn1931
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1931
This article is cited by
-
Interaction between trait and housing condition produces differential decision-making toward risk choice in a rat gambling task
Scientific Reports (2017)
-
Amphetamine Exposure Selectively Enhances Hippocampus-Dependent Spatial Learning and Attenuates Amygdala-Dependent Cue Learning
Neuropsychopharmacology (2010)
-
Impairments of Probabilistic Response Reversal and Passive Avoidance Following Catecholamine Depletion
Neuropsychopharmacology (2009)
-
Switching on and off fear by distinct neuronal circuits
Nature (2008)