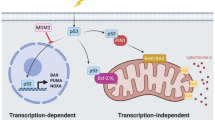
Key Points
-
The ARF tumour suppressor interferes with the MDM2 E3 ubiquitin protein ligase to stabilize and activate the p53 transcription factor, triggering cell-cycle arrest or apoptosis.
-
It is widely accepted that the activity of ARF is mediated through the activation of the p53 transcription programme, but several lines of evidence, much of it controversial, indicate that ARF also exerts p53-independent tumour-suppressor functions.
-
The ARF protein has an unusual amino-acid composition, being highly basic (pI>12, despite a paucity of lysine residues); it is probably unstructured unless bound to other targets and highly promiscuous in its binding.
-
ARF is a nucleolar protein that assembles into high-molecular-mass complexes with nucleophosmin (NPM). Its binding to NPM inhibits ARF turnover and results in its accumulation within the nucleolus.
-
ARF has an associated sumoylating activity that can lead to the modification of proteins to which it binds, including MDM2 and NPM.
-
ARF has now been reported to physically interact with more than 25 other proteins, at least some of which have been postulated to be responsible for its p53-independent functions. These include proteins involved in ribosome biogenesis, transcriptional regulation, the DNA-damage response, apoptosis and autophagy. How strong are the data?
Abstract
Mammalian cells that sustain oncogenic insults can invoke defensive programmes that either halt their division or trigger their apoptosis, but these countermeasures must be finely tuned to discriminate between physiological and potentially harmful growth-promoting states. By functioning specifically to oppose abnormally prolonged and sustained proliferative signals produced by activated oncogenes, the ARF tumour suppressor antagonizes functions of MDM2 to induce protective responses that depend on the p53 transcription factor and its many target genes. However, ARF has been reported to physically associate with proteins other than MDM2 and to have p53-independent activities, most of which remain controversial and poorly understood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003).
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell cycle control causing specific inhibition of cyclin D/CDK4. Nature 366, 704–707 (1993).
Quelle, D. E., Zindy, F., Ashmun, R. A. & Sherr, C. J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Pomerantz, J. et al. The Ink4a tumor suppressor gene product, p19Arf interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92, 713–723 (1998).
Zhang, Y., Xiong, Y. & Yarbrough, W. G. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppressor pathways. Cell 92, 725–734 (1998).
Kamijo, T. et al. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl Acad. Sci. USA 95, 8292–8297 (1998).
Stott, F. J. et al. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 17, 5001–5014 (1998).
Kim, S. H., Mitchell, M., Fujii, H., Llanos, S. & Peters, G. Absence of p16INK4a and truncation of ARF tumor suppressors in chickens. Proc. Natl Acad. Sci. USA 100, 211–216 (2003).
Quelle, D. E., Cheng, M., Ashmun, R. A. & Sherr, C. J. Cancer-associated mutations at the INK4a locus cancel cell cycle arrest by p16INK4a but not by the alternative reading frame protein p19ARF. Proc. Natl Acad. Sci. USA 94, 669–673 (1997).
Gil, J. & Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nature Rev. Mol. Cell. Biol. 7, 667–677 (2006). This recent review summarizes how the genes at the INK4b–ARF–INK4a locus are regulated both positively and negatively in response to different physiological states.
Weber, J. D. et al. p53-independent functions of the p19ARF tumor suppressor. Genes Dev. 14, 2358–2365 (2000). This description of the greatly increased tumour spectrum observed in TKO mice that lack Arf, Mdm2 and Trp53 provided the first evidence that p19ARF might exert tumour-suppressor functions independently of the MDM2–p53 axis.
Kelly-Spratt, K. S., Gurley, K. E., Yasui, Y. & Kemp, C. J. p19Arf suppresses growth, progression, and metastasis of H-ras-driven carcinomas through p53-dependent and-independent pathways. PLoS Biology 2, 1138–1149 (2004). This paper documents the different tumour-suppressor activities of p53 and p19ARF in a mouse model of carcinogen-induced skin cancer.
McKeller, R. N. et al. The Arf tumor suppressor gene promotes hyaloid vascular regression during mouse eye development. Proc. Natl Acad. Sci. USA 99, 3848–3853 (2002).
Silva, R. L. et al. Arf-dependent regulation of Pdgf signaling in perivascular cells in the developing eye. EMBO J. 24, 2803–2814 (2005). The authors argue that ARF regulates PDGF receptor expression and perivascular cell proliferation in the vitreous of the mouse eye in a manner that seems to be independent of p53. This is the first and only clear description of a functional role for ARF in normal development outside the context of tumour suppression.
Carnero, A., Hudson, J. D., Price, C. M. & Beach, D. H. p16INK4a and p19ARF act in overlapping pathways in cellular immortalization. Nature Cell Biol. 2, 148–155 (2000).
Eymin, B. et al. Human ARF binds E2F1 and inhibits its transcriptional activity. Oncogene 20, 1033–1041 (2001).
Yarbrough, W. G., Bessho, M., Zanation, A., Bisi, J. E. & Xiong, Y. Human tumor suppressor ARF impedes S-phase progression independent of p53. Cancer Res. 62, 1171–1177 (2002).
Tsuji, K. et al. p53-independent apoptosis is induced by the p19ARF tumor suppressor. Biochem. Biophys. Res. Comm. 295, 621–629 (2002).
Hemmati, P. G. et al. Adenovirus-mediated overexpression of p14(ARF) induces p53 and Bax-independent apoptosis. Oncogene 21, 3149–3161 (2002).
Eymin, B., Leduc, C., Coll, J. L., Brambilla, E. & Gazzeri, S. p14ARF induces G2 arrest and apoptosis independently of p53 leading to regression of tumours established in nude mice. Oncogene 22, 1822–1835 (2003).
Weber, J. D. et al. Cooperative signals governing ARF-Mdm2 interaction and nucleolar localization of the complex. Mol. Cell. Biol. 20, 2517–2528 (2000).
Xirodimas, D. P., Chisholm, J., Desterro, J. M. S., Lane, D. P. & Hay, R. T. p14ARF promotes accumulation of SUMO-1 conjugated (H)Mdm2. FEBS Lett. 528, 207–211 (2002). This is the first of several papers (references 23 – 27 ) showing that ARF overexpression can trigger protein sumoylation. The underlying mechanism and functional significance of these findings remain unclear.
Chen, L. & Chen, J. MDM2-ARF complex regulates p53 sumoylation. Oncogene 22, 5348–5357 (2003).
Woods, Y. L. et al. p14ARF promotes small ubiquitin-like modifier conjugation of Werners helicase. J. Biol. Chem. 279, 50157–50166 (2004). This study suggests that ARF-induced sumoylation of the WRN helicase might have a role in relocalizing the protein from the nucleolus into the nucleoplasm. It is the only investigation to date that implicates ARF-induced sumoylation in a defined biological process.
Rizos, H., Woodruff, S. & Kefford, R. F. p14ARF interacts with the SUMO-conjugating enzyme Ubc9 and promotes the sumoylation of its binding partners. Cell Cycle 4, 597–603 (2005).
Tago, K., Chiocca, S. & Sherr, C. J. Sumoylation induced by the Arf tumor suppressor: a p53-independent function. Proc. Natl Acad. Sci. USA 102, 7689–7694 (2005).
Johnson, E. S. Protein modification by SUMO. Annu. Rev. Biochem. 73, 355–382 (2004).
Melchior, F. SUMO- nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16, 591–626 (2000).
Itahana, K. et al. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosomal biogenesis and cell proliferation. Mol. Cell. 12, 1151–1164 (2003).
Bertwistle, D., Sugimoto, M. & Sherr, C. J. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 24, 985–996 (2004).
Brady, S. N., Yu, Y., Maggi, L. B. Jr. & Weber, J. D. ARF impedes NPM/B23 shuttling in an Mdm2-sensitive tumor suppressor pathway. Mol. Cell. Biol. 24, 9327–9338 (2004). Whereas references 30, 31 , and 33 also document interactions between ARF and NPM, this paper argues that ARF has a nucleolar function, affecting NPM activity by impeding its shuttling from the nucleolus to the cytoplasm.
Korgaonkar, C. et al. Nucleophosmin (B23) targets ARF to nucleoli and inhibits its function. Mol. Cell. Biol. 25, 1258–1271 (2005). In contrast to reference 32 , the authors suggest that NPM retains ARF in nucleoli, restricting its potential for interacting with nucleoplasmic MDM2.
Hingorani, K., Szebeni, A. & Olson, M. O. Mapping the functional domains of nucleolar protein B23. J. Biol. Chem. 275, 24451–24457 (2000).
Borer, R. A., Lehner, C. F., Eppenberger, H. M. & Nigg, E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 103, 379–390 (1989).
Grisendi, S., Mecucci, C., Falini, B. & Pandolfi, P. P. Nucleophosmin and cancer. Nature Rev. Cancer 6, 493–505 (2006). This encyclopaedic and usefully referenced review discusses the many activities attributed to NPM and their potential contributions to cancer.
Szebani, A. & Olson, M. O. Nucleolar protein B23 has molecular chaperone activities. Protein Sci. 8, 905–912 (1999).
Grisendi, S. et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 437, 147–153 (2005).
Columbo, E. et al. Nucleophosmin is required for DNA integrity and p19Arfprotein stability. Mol. Cell. Biol. 25, 8874–8886 (2005). References 38 and 39 document the pleiotropic effects of disabling the Npm1 gene in the mouse. Given the many different functions ascribed to NPM, it might not be surprising that it is essential for mouse development.
Rodway, H., Llanos, S., Rowe, J. & Peters, G. Stability of nucleolar versus non-nucleolar forms of human p14(ARF). Oncogene 23, 6186–6192 (2004).
Kuo, M. -L., den Besten, W., Bertwistle, D., Roussel, M. F. & Sherr, C. J. N-terminal polyubiquitination and degradation of the Arf tumor suppressor. Genes Dev. 18, 1862–1874 (2004). Metabolic labelling with [3H]-leucine was used to determine the half-life of human and mouse ARF proteins. This study shows the effects of NPM on ARF stabilization, and demonstrates that ARF proteins are polyubiquitylated at their N termini.
Goldberg, A. L. Protein degradation and protection against misfolded or damaged proteins. Nature 426, 895–899 (2003). The author argues that the mistranslation of proteins is frequent, and that mechanisms are in place that recognize improperly folded polypeptides and degrade them, even in the absence of their ubiquitylation.
Ayrault, O., Andrique, L., Larsen, C. J. & Seite, P. Human Arf tumor suppressor specifically interacts with chromatin containing the promoter of rRNA genes. Oncogene 23, 8097–8104 (2004).
Sugimoto, M., Kuo, M. L., Roussel, M. F. & Sherr, C. J. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol. Cell. 11, 415–424 (2003).
Yu, Y. et al. Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol. Cell. Biol. 26, 3798–3809 (2006).
Rubbi, C. P. & Milner, J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 22, 6068–6077 (2003). Disrupting nucleolar integrity is sufficient to induce p53 (see also reference 47).
Pestov, D. G., Strezoska, Z. & Lau, L. F. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G1/S transition. Mol. Cell. Biol. 22, 4246–4255 (2001). Similar to the conclusions of reference 46 , a mutant protein that interferes with rRNA processing triggers a p53 response.
Budde, A. & Grummt, I. p53 represses ribosomal gene transcription. Oncogene 28, 1119–1124 (1999).
Zhai, W. & Comai, L. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol. Cell. Biol. 20, 5930–5938 (2000).
Llanos, S., Clark, P. A., Rowe, J. & Peters, G. Stabilization of p53 by p14ARF without relocation of MDM2 to the nucleolus. Nature Cell. Biol. 3, 445–452 (2001).
Gjerset, R. A. & Bandyopadhyay, K. Regulation of p14ARF through subnuclear compartmentalization. Cell Cycle 5, 686–690 (2006).
Weber, J. D., Taylor, L. J., Roussel, M. F., Sherr, C. J. & Bar-Sagi, D. Nucleolar Arf sequesters Mdm2 and activates p53. Nature Cell Biol. 1, 20–26 (1999).
Tao, W. & Levine, A. J. P19ARF stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl Acad. Sci. USA 96, 6937–6941 (1999).
Lohrum, M. A. E., Ashcroft, M., Kubbutat, M. H. G. & Vousden, K. H. Identification of a cryptic nucleolar-localization signal in MDM2. Nature Cell Biol. 2, 179–181 (2000).
Bracken, A. P., Ciro, M., Cocito, A. & Helin, K. E2F target genes: unraveling the biology. Trends Biochem. Sci. 29, 409–417 (2004).
Blais, A. & Dynlacht, B. D. Hitting their targets: an emerging picture of E2F and cell cycle control. Curr. Opin. Genet. Dev. 14, 527–532 (2004). These two reviews point to an extensive network of genes governed by E2F transcription factors, many of which are implicated in regulating activities other than the G1–S transition. Both papers highlight the power of 'ChIP on chip' technologies in studying promoter occupancy.
Rowland, B. D. et al. E2F transcriptional repressor complexes are critical downstream targets of p19ARF/p53-induced proliferative arrest. Cancer Cell 2, 55–65 (2002).
Aslanian, A., Iaquinta, P. J., Verona, R. & Lees, J. A. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18, 1413–1422 (2004).
Komori, H., Enomoto, M., Nakamura, M., Iwanaga, R. & Ohtani, K. Distinct E2F-mediated transcriptional program regulates p14ARF gene expression. EMBO J. 24, 3724–3736 (2005).
Martelli, F. et al. p19ARF targets certain E2F species for degradation. Proc. Natl Acad. Sci. USA. 98, 4455–4460 (2001).
Datta, A., Nag, A. & Raychaudhuri, P. Differential regulation of E2F1, DP1, and the E2F1/DP1 complex by ARF. Mol. Cell. Biol. 22, 8398–8408 (2002).
Datta, A. et al. ARF directly binds DP1: interaction with DP1 coincides with the G1 arrest function of ARF. Mol. Cell. Biol. 25, 8024–8036 (2005). This is the latest in a series of papers from this group that highlight direct physical interactions between E2F subunits and ARF.
Qi, Y. et al. p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature 431, 712–717 (2004). This provocative report provides experimental support for the idea that ARF interacts with MYC-containing transcriptional complexes on chromatin to regulate transactivation but not transrepression.
Datta, A. et al. Myc-ARF (alternative reading frame) interaction inhibits the functions of Myc. J. Biol. Chem. 279, 36698–36707 (2004).
Gregory, M. A., Qi, Y. & Hann, S. R. The ARF tumor suppressor: keeping Myc on a leash. Cell Cycle 4, 249–252 (2005).
Shiio, Y. & Eisenman, R. N. Histone sumoylation is associated with transcriptional repression. Proc. Natl Acad. Sci. USA 100, 13118–13120 (2003).
Chen, D. et al. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell 121, 1071–1083 (2005). ARF binding to ARF-BP1 (also known as HectH9 and MULE) inhibits its ubiquitin E3 ligase activity directed at p53. The same protein has been independently found to ubiquitylate MYC (see reference 68 ) and the cytoplasmic BCL2 family member MCL1 (see reference 106).
Adhikary, S. et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 123, 409–421 (2005). See reference 67
Frank, S. R. et al. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 4, 575–580 (2003).
Amente, S. et al. p14ARF directly interacts with Myc through the Myc BOX II domain. Cancer Biol. Ther. 5, 287–291 (2006).
Eymin, B. et al. p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol. Cell. Biol. 26, 4339–4350 (2006).
Leduc, C. et al. p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation. Oncogene (2006) (in the press).
Kalinichenko, V. V. et al. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 18, 830–850 (2004).
Costa, R. H., Kalinichenko, V. V., Major, M. L. & Raychaudhuri, P. New and unexpected: forkhead meets ARF. Curr. Opin. Genet. Dev. 15, 42–48 (2005).
Suzuki, H. et al. The ARF tumor suppressor inhibits BCL6-mediated transcriptional repression. Biochem. Biophys. Res. Comm. 326, 242–248 (2005).
Shieh, S. -Y., Ikeda, M., Taya, Y. & Prives, P. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 (1997).
Siliciano, J. D. et al. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11, 3471–3481 (1997).
Li, Y. et al. ATM activity contributes to the tumor-suppressing functions of p14(ARF). Oncogene 23, 7355–7365 (2004).
Khan, S., Guevera, C., Fujii, G. & Parry, D. p14ARF is a component of the p53 response following ionizing irradiation of normal human fibroblasts. Oncogene 23, 6040–6046 (2004).
Pauklin, S., Kristjuhan, A., Maimets, T. & Jaks, V. ARF and ATM/ATR cooperate in p53-mediated apoptosis upon oncogenic stress. Biochem. Biophys. Res. Commun. 334, 386–394 (2005).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005). References 81 and 82 show that oncogenic stress causes DNA damage in early precancerous lesions. The induced checkpoint responses play a tumour-suppressor role in preventing or delaying human cancer.
Pusapati, R. V. et al. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc. Natl Acad. Sci. USA 103, 1446–1451 (2006).
Parrinello, S. et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biol. 5, 741–747 (2003). Primary cells cultured under low oxygen tension (more closely approximating their physiological state in vivo ) overcome 'culture shock' and have an extended lifespan.
Chua, K. F. et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2, 67–76 (2006). Although it is not the central theme of this report, these investigators show that primary MEFs grown in subliminal concentrations of H 2 O 2 activate ARF and undergo premature senescence.
Vafa, O. et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell 9, 1031–1044 (2002). This intriguing paper provides direct evidence that the MYC oncogene can increase reactive oxygen species and induce DNA damage. It complements results reported in reference 88.
Halazonetis, T. D. Constitutively active DNA damage checkpoint pathways as the driving force for the high frequency of p53 mutations in human cancer. DNA Repair 3, 1057–1062 (2004).
Karlsson, A. et al. Defective double-strand DNA break repair and chromosomal translocations by MYC overexpression. Proc. Natl Acad. Sci. USA 96, 3940–3944 (1999). See reference 86.
Rocha, S., Campbell, K. J. & Perkins, N. D. p53- and Mdm2-independent repression of NF-κB transactivation by the ARF tumor suppressor. Mol. Cell 12, 15–25 (2003).
Rocha, S., Garrett, M. D., Campbell, K. J., Schumm, K. & Perkins, N. D. Regulation of NF-κB and p53 through activation of ATR and Chk1 by the ARF tumour suppressor. EMBO J. 24, 1157–1169 (2005).
Rocha, S. & Perkins, N. D. ARF the integrator: linking NF-κB, p53 and checkpoint kinases. Cell Cycle 4, 756–759 (2005).
Sun, Y., Jiang, S., Chen, N., Fernandes, N. & Price, B. D. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl Acad. Sci. USA 102, 13182–13187 (2005).
Khan, S. H., Moritsugu, J. & Wahl, G. M. Differential requirement for p19ARF in the p53-dependent arrest induced by DNA damage, microtubule disruption, and ribonucleotide depletion. Proc. Natl Acad. Sci. USA 97, 3266–3271 (2000).
Lee, C., Smith, B. A., Bandyopadhyay, K. & Gjerset, R. A. DNA damage disrupts the p14ARF-B23 (nucleophosmin) interaction and triggers a transient subnuclear redistribution of p14ARF. Cancer Res. 65, 9834–9842 (2005).
Reef, S. et al. A novel mitochondrial short form of p19ARF induces autophagy and caspase-independent cell death. Mol. Cell 22, 463–475 (2006). Initiation of translation at unique internal methionine residues in human and mouse ARF mRNAs results in the production of N-terminally truncated smARF proteins that lack MDM2 or NPM binding capability. The authors provide provocative evidence that these unstable proteins home to mitochondria and trigger autophagy.
Kondo, Y., Kanazawa, T., Sawaya, R. & Kondo, S. The role of autophagy in cancer development and response to therapy. Nature Rev. Cancer 5, 726–734 (2005).
Lum, J. J., DeBerardinis, R. J. & Thompson, C. B. Autophagy in metazoans: cell survival in the land of plenty. Nature Rev. Mol. Cell. Biol. 6, 439–448 (2005).
Lozano, G. & Zambetti, G. P. What have animal models taught us about the p53 pathway? J. Pathol. 205, 206–220 (2005).
Poyurovsky, M. V. & Prives, C. Unleashing the power of p53: lessons from mice and men. Genes Dev. 20, 125–131 (2006).
Moore, L. V. S. et al. Cooperativity of p19ARF, Mdm2, and p53 in murine tumorigesis. Oncogene 22, 7831–7837 (2003).
Menendez, S. et al. Oligomerization of the human ARF tumor suppressor and its response to oxidative stress. J. Biol. Chem. 278, 18720–18729 (2003).
Falini, B. et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. New Eng. J. Med. 352, 254–266 (2005). This paper documents the surprising occurrence of NPM C-terminal frameshift mutations in more than a third of adult cases of acute myeloid leukaemia (AML), showing these to be the single most common genetic anomally in AML.
den Besten, W., Kuo, M. -L., Williams, R. T. & Sherr, C. J. Myeloid leukemia-associated nucleophosmin mutants perturb p53-dependent and independent activities of the Arf tumor suppressor protein. Cell Cycle 4, 1593–1598 (2005).
Colombo, E. et al. Delocalization and destabilization of the Arf tumor suppressor by the leukemia-associated NPM mutant. Cancer Res. 66, 3044–3050 (2006). References 103 and 104 illustrate that NPMc+ mutants relocalize the bulk of cellular ARF protein from the nucleolus to the cytoplasm. Reference 104 suggests that cytoplasmic ARF–NPM complexes are destabilized, whereas no effect on ARF stability was observed in reference 103.
Cleveland, J. L. & Sherr, C. J. Antagonism of Myc functions by Arf. Cancer Cell 6, 309–311 (2004).
Tompkins, V., Hagen, J., Zediak, V. P. & Quelle, D. E. Identification of novel ARF binding proteins by two-hybrid screening. Cell Cycle 5, 641–646 (2006).
Zhong, Q., Gao, W., Du, F. & Wang, X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085–1095 (2005). See reference 67.
Shvarts, A. et al. A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev. 16, 681–686 (2002).
Hasan, M. K. et al. CARF is a novel protein that cooperates with mouse p19ARF (Human p14ARF) in activating p53. J. Biol. Chem. 277, 37765–37770 (2002).
Wadhwa, R. et al. A novel putative collaborator of p19ARF. Exp. Gerontol. 38, 245–252 (2003).
Hasan, M. K. et al. Alternative reading frame protein (ARF)-independent function of CARF (collaborator of ARF) involves its interactions with p53: evidence for a novel p53-activation pathway and its negative feedback control. Biochem J. 380, 605–610 (2004).
Paliwal, S. et al. Targeting of C-terminal binding protein (CtBP) by ARF results in p53-independent apoptosis. Mol. Cell. Biol. 26, 2360–2372 (2006).
Zhao, L. et al. Cyclin G1 has growth inhibitory activity linked to the ARF-Mdm2-p53 and pRb tumor suppressor pathways. Mol. Cancer Res. 1, 195–206 (2003).
Fatyol, K. & Szalay, A. A. The p14ARF tumor suppressor protein facilitates nucleolar sequestration of hypoxia-inducible factor-1α (HIF-1α) and inhibits HIF-1-mediated transcription. J. Biol. Chem. 276, 28421–28429 (2001).
Pan, W., Datta, A., Adaami, G. R., Raychaudhuri, P. & Bagchi, S. p19ARF inhibits the functions of the HPV16 E7 oncoprotein. Oncogene 22, 5496–5503 (2003).
Wang, J., He, X., Luo, Y. & Yarbrough, W. G. A novel ARF-binding protein (LZAP) alters ARF regulation of HDM2. Biochem J. 393, 489–501 (2006).
Zhang, Y. & Xiong, Y. Control of p53 ubiquitination and nuclear export by MDM2 and ARF. Cell Growth & Differ. 12, 175–186 (2001).
Calabro, V. et al. Inhibition of p63 transcriptional activity by p14ARF: functional and physical link between human ARF tumor suppressor and a member of the p53 family. Mol. Cell. Biol. 24, 8529–8540 (2004).
Rizos, H. et al. Association of p14ARF with the p120E4F transcriptional repressor enhances cell cycle inhibition. J. Biol. Chem. 278, 4981–4989 (2003).
Sugihara, T. et al. Pex19p dampens the p19ARF-p53-p21WAF1 tumor suppressor pathway. J. Biol. Chem. 276, 18649–18652 (2001).
Vivo, M. et al. The human tumor suppressor ARF interacts with spinophilin/neurabin II, a type 1 protein-phosphatase-binding protein. J. Biol. Chem. 276, 14161–14169 (2001).
Pollice, A. et al. Functional and physical interaction of the human ARF tumor suppressor with Tat-binding protein-1. J. Biol. Chem. 279, 6345–6353 (2004).
Karayan, L. et al. Human ARF protein interacts with topoisomerase I and stimulates its activity. Oncogene 20, 836–848 (2001).
Sui, G. et al. Ying Yang 1 is a negative regulator of p53. Cell 117, 859–872 (2004).
Acknowledgements
The author thanks M. B. Kastan, J. L. Cleveland, J. T. Opferman and M. F. Roussel for critical comments and suggestions.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Retinoblastoma protein
-
(RB) Named for its tumour-suppressor role in familial retinoblastoma, RB and its related family members (p130 and p107) function as transcriptional co-repressors to prevent cells from entering the DNA synthesis (S) phase of the cell division cycle.
- Cyclin-dependent kinases
-
(CDKs) These enzymes are composed of a catalytic CDK subunit and a regulatory cyclin subunit. The cyclin D-dependent CDKs are activated in response to extracellular mitogenic signals and phosphorylate the retinoblastoma protein (RB) and other RB-family members (p107 and p130).
- E3 ubiquitin ligase
-
The addition of polyubiquitin chains to proteins is carried out by a cascade of three enzymes, designated E1, E2 and E3, that sequentially activate and transfer ubiquitin. The selection of protein substrates for ubiquitylation is mediated by the E3 enzymes.
- Hyaloid vascular system
-
(HVS) During eye development, this delicate vasculature extends from the optic cup in the back of the eye through the vitreous to supply the lens. In the mouse, the HVS involutes within the first 10 days postnatally, correlating with maximal p19ARF expression in pericytes surrounding the vascular endothelium.
- INK4 proteins
-
So named because they are inhibitors of CDK4, these proteins associate with and block the enzymatic activities of the cyclin D-dependent kinases CDK4 and CDK6. There are four such proteins, designated INK4a, INK4b, INK4c, and INK4d in order of their discovery.
- Tandem-affinity tagging
-
The synthesis of a recombinant protein engineered to include multiple 'tags' (for example, small peptides added to its N or C terminus) enables it to be biochemically recovered through sequential affinity purification steps usually performed using antibodies to the tags.
- Isoelectric point
-
The pH at which dipolar molecules do not migrate in an electrical field. Neutralization of the charge of the highly arginine-rich ARF protein requires a pH >12.
- Sumoylation
-
The small ubiquitin-like modifier (SUMO) is conjugated to target proteins in a manner analogous to ubiquitylation. The biological consequences of sumoylation are relatively poorly understood, although the process might regulate diverse cellular activities such as protein trafficking, chromatin remodelling and gene expression.
- Nucleolus
-
An intranuclear organelle that is the primary site of ribosomal DNA transcription, rRNA processing, ribosome assembly and transport.
- Proteasome
-
A macromolecular 'machine' that hydrolyses proteins into their constituent amino acids.
- MYC
-
MYC is a transcription factor that, following heterodimerization with its partner MAX, can bind directly to canonical hexameric DNA sequences found in the promoters of many genes. Depending on other proteins recruited to promoters, the MYC–MAX complex can either transactivate or repress gene expression.
- Radius of gyration
-
A parameter characterizing the size of a particle of any shape, used in this context to refer to different penultimate amino acids at the N terminus of a polypeptide chain.
- ATR and ATM
-
ATM, the gene mutated in the ataxia-telangiectasia syndrome, encodes a protein kinase whose activity is triggered by DNA double-strand breaks. The ATM and RAD3-related kinase, ATR, has a similar role but is primarily activated by stalled replication forks.
- Nuclear factor κB
-
A family of transcription factors that regulate the inflammatory response. Some NFκB family members interfere with apoptosis.
- γ-H2AX
-
A modified histone H2 variant, the phosphorylation of which occurs at chromosomal sites of DNA damage.
Rights and permissions
About this article
Cite this article
Sherr, C. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer 6, 663–673 (2006). https://doi.org/10.1038/nrc1954
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc1954
This article is cited by
-
Tau protein binds to the P53 E3 ubiquitin ligase MDM2
Scientific Reports (2023)
-
CRL2-KLHDC3 E3 ubiquitin ligase complex suppresses ferroptosis through promoting p14ARF degradation
Cell Death & Differentiation (2022)
-
Targeting ferroptosis as a vulnerability in cancer
Nature Reviews Cancer (2022)
-
Nucleus-translocated mitochondrial cytochrome c liberates nucleophosmin-sequestered ARF tumor suppressor by changing nucleolar liquid–liquid phase separation
Nature Structural & Molecular Biology (2022)
-
HO-1 nuclear accumulation and interaction with NPM1 protect against stress-induced endothelial senescence independent of its enzymatic activity
Cell Death & Disease (2021)