Key Points
-
Cisplatin is a platinum-based drug that has been in widespread use for many years to treat several forms of cancer, including testicular, ovarian, cervical, head and neck, and non-small-cell lung cancer.
-
Treatment is limited, however, by side effects including nephrotoxicity, emetogenesis and neurotoxicity. In addition, both inherent and acquired resistance to the drug limits its applications.
-
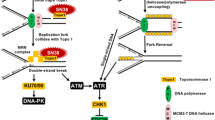
A large body of knowledge on the cellular processing of cisplatin has now emerged. This information has afforded a much more detailed understanding of how cisplatin-induced DNA damage is recognized, how the damage signals are transduced, how the cell-cycle arrests, and how DNA repair and apoptosis are activated.
-
This knowledge provides us with a basis for developing improved therapeutic strategies exploiting platinum-based drugs and/or the rational design of new platinum-based compounds. Disruptions of certain pathways by inhibitors, activators or combinations of drugs that modulate cellular sensitivity to cisplatin are likely to lead to clinical benefit.
Abstract
Cisplatin, carboplatin and oxaliplatin are platinum-based drugs that are widely used in cancer chemotherapy. Platinum–DNA adducts, which are formed following uptake of the drug into the nucleus of cells, activate several cellular processes that mediate the cytotoxicity of these platinum drugs. This review focuses on recently discovered cellular pathways that are activated in response to cisplatin, including those involved in regulating drug uptake, the signalling of DNA damage, cell-cycle checkpoints and arrest, DNA repair and cell death. Such knowledge of the cellular processing of cisplatin adducts with DNA provides valuable clues for the rational design of more efficient platinum-based drugs as well as the development of new therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bosl, G. J., Bajorin, D. F. & Sheinfeld, J. Cancer of the Testis (eds DeVita, V. T. J., Hellman, S. & Rosenberg, S. A.) (Lippincott Williams & Wilkins, Philadelphia, 2001).
Jamieson, E. R. & Lippard, S. J. Structure, recognition, and processing of cisplatin–DNA adducts. Chem. Rev. 99, 2467–2498 (1999). This comprehensive review details structural studies of cisplatin–DNA adducts and describes cellular proteins that recognize cisplatin–DNA adducts.
Wong, E. & Giandomenico, C. M. Current status of platinum-based antitumor drugs. Chem. Rev. 99, 2451–2466 (1999).
Decatris, M. P., Sundar, S. & O'Byrne, K. J. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat. Rev. 30, 53–81 (2004). This recent review describes cisplatin-/carboplatin-based regimens combined with many other cytotoxic drugs in the treatment of metastatic breast cancer.
Kelland, L. R. et al. Discovery and development of platinum complexes designed to circumvent cisplatin resistance. J. Inorg. Biochem. 77, 111–115 (1999).
Fuertes, M. A., Alonso, C. & Pérez, J. -M. Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem. Rev. 103, 645–662 (2003). This review article details the molecular bases of cisplatin resistance and biochemical modulation strategies directed to circumvent cisplatin resistance.
Chaney, S. G., Campbell, S. L., Bassett, E. & Wu, Y. Recognition and processing of cisplatin- and oxaliplatin–DNA adducts. Crit. Rev. Oncol. Hematol. 53, 3–11 (2005).
Rixe, O. et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem. Pharmacol. 52, 1855–1865 (1996).
Ho, Y. P., Au-Yeung, S. C. & To, K. K. Platinum-based anticancer agents: innovative design strategies and biological perspectives. Med. Res. Rev. 23, 633–655 (2003).
Barnes, K. R. & Lippard, S. J. in Metal Ions in Biological Systems. (ed. Sigel, H.) 143–177 (Marcel Dekker, New York, 2004).
Natile, G. & Coluccia, M. in Metal Ions in Biological Systems. (ed. Sigel, H.) 209–249 (Marcel Dekker, New York, 2004).
Perez, J. M., Fuertes, M. A., Alonso, C. & Navarro-Ranninger, C. Current status of the development of trans-platinum antitumor drugs. Crit. Rev. Oncol. Hematol. 35, 109–120 (2000).
Barnes, K. R., Kutikov, A. & Lippard, S. J. Synthesis, characterization, and cytotoxicity of a series of estrogen-tethered platinum(IV) complexes. Chem. Biol. 11, 557–564 (2004).
Hromas, R. A., North, J. A. & Burns, C. P. Decreased cisplatin uptake by resistant L1210 leukemia cells. Cancer Lett. 36, 197–201 (1987).
Mann, S. C., Andrews, P. A. & Howell, S. B. Modulation of cis-diamminedichloroplatinum(II) accumulation and sensitivity by forskolin and 3-isobutyl-1-methylxanthine in sensitive and resistant human ovarian carcinoma cells. Int. J. Cancer 48, 866–872 (1991).
Binks, S. P. & Dobrota, M. Kinetics and mechanism of uptake of platinum-based pharmaceuticals by the rat small intestine. Biochem. Pharmacol. 40, 1329–1336 (1990).
Ishida, S., Lee, J., Thiele, D. J. & Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl Acad. Sci. USA 99, 14298–14302 (2002). This pioneering paper identifies an unexpected pathway for uptake of cisplatin and establishes Ctr1 as a major component in ushering cisplatin into mammalian cells. It points to a potentially critical protein for sensitizing cells to the drug.
Holzer, A. K. et al. The copper influx transporter human copper transport protein 1 regulates the uptake of cisplatin in human ovarian carcinoma cells. Mol. Pharmacol. 66, 817–823 (2004).
Komatsu, M. et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 60, 1312–1316 (2000).
Miyashita, H. et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker in human oral squamous cell carcinoma treated with cisplatin. Oral Oncol. 39, 157–162 (2003).
Nakayama, K. et al. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin. Cancer Res. 10, 2804–2811 (2004).
Nakayama, K. et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) as a cisplatin based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int. J. Cancer 101, 488–495 (2002).
Ohbu, M. et al. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human gastric carcinoma. Cancer Lett. 189, 33–38 (2003).
Cui, Y. et al. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 55, 929–937 (1999).
Kool, M. et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 57, 3537–3547 (1997).
Koike, K. et al. A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res. 57, 5475–5479 (1997).
Ohashi, K. et al. Copper(II) protects yeast against the toxicity of cisplatin independently of the induction of metallothionein and the inhibition of platinum uptake. Biochem. Biophys. Res. Comm. 310, 148–152 (2003).
Katano, K. et al. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 62, 6559–6565 (2002).
Safaei, R. et al. Cross-resistance to cisplatin in cells with acquired resistance to copper. Cancer Chemother. Pharmacol. 53, 239–246 (2004).
Harrap, K. R. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat. Rev. 12 (Suppl. A), 21–33 (1985).
Tashiro, T., Kawada, Y., Sakurai, Y. & Kidani, Y. Antitumor activity of a new platinum complex, oxalato (trans-l-1,2-diaminocyclohexane)platinum (II): new experimental data. Biomed. Pharmacother. 43, 251–260 (1989).
Chaney, S. G. et al. Protein interactions with platinum–DNA adducts: from structure to function. J. Inorg. Biochem. 98, 1551–1559 (2004).
Raymond, E., Faivre, S., Chaney, S., Woynarowski, J. & Cvitkovic, E. Cellular and molecular pharmacology of oxaliplatin. Mol. Cancer Ther. 1, 227–235 (2002).
Jung, Y. & Lippard, S. J. Multiple states of stalled T7 RNA polymerase at DNA lesions generated by platinum anticancer agents. J. Biol. Chem. 278, 52084–52092 (2003).
Vaisman, A. et al. Effect of DNA polymerases and high mobility group protein 1 on the carrier ligand specificity for translesion synthesis past platinum–DNA adducts. Biochemistry 38, 11026–11039 (1999).
Zdraveski, Z. Z., Mello, J. A., Farinelli, C. K., Essigmann, J. M. & Marinus, M. G. MutS preferentially recognizes cisplatin- over oxaliplatin-modified DNA. J. Biol. Chem. 277, 1255–1260 (2002).
Kartalou, M. & Essigmann, J. M. Recognition of cisplatin adducts by cellular proteins. Mutat. Res. 478, 1–21 (2001).
Wozniak, K. & Blasiak, J. Recognition and repair of DNA–cisplatin adducts. Acta Biochim. Pol. 49, 583–596 (2002).
Swanson, P. C. Fine structure and activity of discrete RAG-HMG complexes on V(D)J recombination signals. Mol. Cell. Biol. 22, 1340–1351 (2002).
Aidinis, V. et al. The RAG1 homeodomain recruits HMG1 and HMG2 to facilitate recombination signal sequence binding and to enhance the intrinsic DNA-bending activity of RAG1-RAG2. Mol. Cell. Biol. 19, 6532–6542 (1999).
van Gent, D. C., Hiom, K., Paull, T. T. & Gellert, M. Stimulation of V(D)J cleavage by high mobility group proteins. EMBO J. 16, 2665–2670 (1997).
Yuan, F., Gu, L., Guo, S., Wang, C. & Li, G. -M. Evidence for involvement of HMGB1 protein in human DNA mismatch repair. J. Biol. Chem. 279, 20935–20940 (2004).
Jayaraman, L. et al. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 12, 462–472 (1998).
Imamura, T. et al. Interaction with p53 enhances binding of cisplatin-modified DNA by high mobility group 1 protein. J. Biol. Chem. 276, 7534–7540 (2001).
Huang, J. -C., Zamble, D. B., Reardon, J. T., Lippard, S. J. & Sancar, A. HMG-domain proteins specifically inhibit the repair of the major DNA adduct of the anticancer drug cisplatin by human excision nuclease. Proc. Natl Acad. Sci. USA 91, 10394–10398 (1994). This paper demonstrates that HMG-domain proteins HMG1 and human mitochondrial transcription factor specifically inhibit repair of the cisplatin 1,2-intrastrand cross-link by the human excision nuclease. The results suggest that the types and levels of HMG-domain proteins in a given tumour might influence its responsiveness to cisplatin and provide a rational basis for the synthesis of new platinum anticancer drug candidates.
Zamble, D. B., Mu, D., Reardon, J. T., Sancar, A. & Lippard, S. J. Repair of cisplatin–DNA adducts by the mammalian excision nuclease. Biochemistry 35, 10004–10013 (1996).
Zamble, D. B., Mikata, Y., Eng, C. H., Sandman, K. E. & Lippard, S. J. Testis-specific HMG-domain protein alters the responses of cells to cisplatin. J. Inorg. Biochem. 91, 451–462 (2002).
He, Q., Liang, C. H. & Lippard, S. J. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proc. Natl Acad. Sci. USA 97, 5768–5772 (2000). This paper describes that treatment of human cancer cells having steroid hormone receptors with the appropriate hormone, oestrogen and/or progesterone, significantly increases the potency of cisplatin and its analogue carboplatin by causing the overexpression of HMGB1.
Brown, S. J., Kellett, P. J. & Lippard, S. J. Ixr1, a yeast protein that binds to platinated DNA and confers sensitivity to cisplatin. Science 261, 603–605 (1993).
McA'Nulty, M. M. & Lippard, S. J. The HMG-domain protein Ixr1 blocks excision repair of cisplatin–DNA adducts in yeast. Mutat. Res. 362, 75–86 (1996).
Nagatani, G. et al. Transcriptional activation of the human HMG1 gene in cisplatin-resistant human cancer cells. Cancer Res. 61, 1592–1597 (2001).
Wei, M., Burenkova, O. & Lippard, S. J. Cisplatin sensitivity in Hmbg1−/− and Hmbg1+/+ mouse cells. J. Biol. Chem. 278, 1769–1773 (2003).
Kunz, C., Zurbriggen, K. & Fleck, O. Mutagenesis of the HMGB (high-mobility group B) protein Cmb1 (cytosine-mismatch binding 1) of Schizosaccharomyces pombe: effects on recognition of DNA mismatches and damage. Biochem. J. 372, 651–660 (2003).
Pallier, C. et al. Association of chromatin proteins high mobility group box (HMGB) 1 and HMGB2 with mitotic chromosomes. Mol. Biol. Cell 14, 3414–3426 (2003).
Bonaldi, T., Längst, G., Strohner, R., Becker, P. B. & Bianchi, M. E. The DNA chaperone HMGB1 facilitates ACF/CHRAC-dependent nucleosome sliding. EMBO J. 21, 6865–6873 (2002). This paper describes that transient interaction of HMGB1 with nucleosomal linker DNA overlapping ISWI-binding sites enhances the ability of ACF to bind nucleosomal DNA and accelerates the sliding activity of limiting concentrations of remodelling factor.
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002). This paper reveals that Hmgb1−/− necrotic cells have a greatly reduced ability to promote inflammation, which proves that the release of HMGB1 can signal the demise of a cell to its neighbors. In apoptotic cells, HMGB1 is bound firmly to chromatin.
Degryse, B. & de Virgilio, M. The nuclear protein HMGB1, a new kind of chemokine? FEBS Lett. 553, 11–17 (2003).
Bianchi, M. E. & Manfredi, A. Chromatin and cell death. Biochim. Biophys. Acta 1677, 181–186 (2004).
Bustin, M. At the crossroads of necrosis and apoptosis: signaling to multiple cellular targets by HMGB1. Science's STKE [online], <http://www.stke.sciencemag.org/cgi/content/full/OC_sigtrans;2002/151/pe39> (2002).
Bruhn, S. L., Pil, P. M., Essigmann, J. M., Housman, D. E. & Lippard, S. J. Isolation and characterization of human cDNA clones encoding a high mobility group box protein that recognizes structural distortions to DNA caused by binding of the anticancer agent cisplatin. Proc. Natl Acad. Sci. USA 89, 2307–2311 (1992). Human cDNA clones encoding a structure-specific recognition protein, SSRP1, that binds specifically to DNA modified with cisplatin are isolated and characterized in this paper.
Saunders, A. et al. Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science 301, 1094–1096 (2003).
Orphanides, G., Wu, W. -H., Lane, W. S., Hampsey, M. & Reinberg, D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature 400, 284–288 (1999).
Yarnell, A. T., Oh, S., Reinberg, D. & Lippard, S. J. Interaction of FACT, SSRP1, and the high mobility group (HMG) domain of SSRP1 with DNA damaged by the anticancer drug cisplatin. J. Biol. Chem. 276, 25736–25741 (2001).
Franke, T. F. et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 (1995).
Pommier, Y., Sordet, O., Antony, S., Hayward, R. L. & Kohn, K. W. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 23, 2934–2949 (2004).
Datta, S. R., Brunet, A. & Greenberg, M. E. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999).
Fraser, M. et al. p53 Is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 63, 7081–7088 (2003).
Dan, H. C. et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J. Biol. Chem. 279, 5405–5412 (2004).
Asselin, E., Mills, G. B. & Tsang, B. K. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 61, 1862–1868 (2001).
Barthwal, M. K. et al. Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J. Biol. Chem. 27, 3897–3902 (2003).
Kim, A. H., Khursigara, G., Sun, X., Franke, T. F. & Chao, M. V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21, 893–901 (2001).
Viniegra, J. G. et al. Modulation of PI3K/Akt pathway by E1a mediates sensitivity to cisplatin. Oncogene 21, 7131–7136 (2002).
Mabuchi, S. et al. Inhibition of NF-κB Increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J. Biol. Chem. 279, 23477–23485 (2004).
Shaul, Y. c-Abl: activation and nuclear targets. Cell Death Differ. 7, 10–16 (2000).
Theis, S. & Roemer, K. c-Abl tyrosine kinase can mediate tumor cell apoptosis independently of the Rb and p53 tumor suppressors. Oncogene 17, 557–564 (1998).
Cong, F. & Goff, S. P. c-Abl-induced apoptosis, but not cell cycle arrest, requires mitogen-activated protein kinase kinase 6 activation. Proc. Natl Acad. Sci. USA 96, 13819–13824 (1999).
Gong, J. et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399, 806–809 (1999).
Machuy, N., Rajalingam, K. & Rudel, T. Requirement of caspase-mediated cleavage of c-Abl during stress-induced apoptosis. Cell Death Differ. 11, 290–300 (2004).
Wang, J. Y. J. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 19, 5643–5650 (2000). This comprehensive review describes a role for the nuclear c-Abl tyrosine kinase in the regulation of cell cycle checkpoints and DNA repair and apoptosis.
Agami, R., Blandino, G., Oren, M. & Shaul, Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature 399, 809–813 (1999).
Yuan, Z. -M. et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature 399, 814–817 (1999).
Sionov, R. V. et al. c-Abl neutralizes the inhibitory effect of Mdm2 on p53. J. Biol. Chem. 274, 8371–8374 (1999).
Kharbanda, S. et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376, 785–788 (1995).
Kharbanda, S., Yuan, Z. -M., Weichselbaum, R. & Kufe, D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene 17, 3309–3318 (1998).
Sanchez-Prieto, R., Sanchez-Arevalo, V. J., Servitja, J. -M. & Gutkind, J. S. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene 21, 974–979 (2002).
Kharbanda, S. et al. Activation of MEK kinase 1 by the c-Abl protein tyrosine kinase in response to DNA damage. Mol. Cell. Biol. 20, 4979–4989 (2000).
Pandey, P. et al. Activation of p38 mitogen-activated protein kinase by c-Abl-dependent and-independent mechanisms. J. Biol. Chem. 271, 23775–23779 (1996).
David-Cordonnier, M. -H. et al. The DNA-binding domain of human c-Abl tyrosine kinase promotes the interaction of a HMG chromosomal protein with DNA. Nucleic Acids Res. 27, 2265–2270 (1999).
Miao, Y. -J. & Wang, J. Y. J. Binding of A/T-rich DNA by three high mobility group-like domains in c-Abl tyrosine kinase. J. Biol. Chem. 271, 22823–22830 (1996).
Hollstein, M., Sidransky, D., Vogelstein, B. & Harris, C. C. p53 mutations in human cancers. Science 253, 49–53 (1991).
Oren, M. Decision making by p53: life, death and cancer. Cell Death Differ. 10, 431–442 (2003).
Geske, F. J. et al. DNA repair is activated in early stages of p53-induced apoptosis. Cell Death Differ. 7, 393–401 (2000).
Benchimol, S. p53-dependent pathways of apoptosis. Cell Death Differ. 8, 1049–1051 (2001).
McKay, B. C., Ljungman, M. & Rainbow, A. J. Potential roles for p53 in nucleotide excision repair. Carcinogenesis 20, 1389–1396 (1999).
Seo, Y. R., Fishel, M. L., Amundson, S., Kelley, M. R. & Smith, M. L. Implication of p53 in base excision DNA repair: in vivo evidence. Oncogene 21, 731–737 (2002).
Adimoolam, S. & Ford, J. M. p53 and regulation of DNA damage recognition during nucleotide excision repair. DNA Repair 2, 947–954 (2003).
Adimoolam, S. & Ford, J. M. p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc. Natl Acad. Sci. USA 99, 12985–12990 (2002).
Wang, D. & Lippard, S. J. Cisplatin-induced post-translational modification of histones H3 and H4. J. Biol. Chem. 279, 20622–20625 (2004).
Wang, X. W. et al. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nature Genet. 10, 188–195 (1995).
Dutta, A., Ruppert, J. M., Aster, J. C. & Winchester, E. Inhibition of DNA replication factor RPA by p53. Nature 365, 79–82 (1993).
Vekris, A. et al. Molecular determinants of the cytotoxicity of platinum compounds: the contribution of in silico research. Cancer Res. 64, 356–362 (2004).
Kanamori, Y. et al. A newly developed adenovirus-mediated transfer of a wild-type p53 gene increases sensitivity to cis-diamminedichloroplatinum (II) in p53-deleted ovarian cancer cells. Eur. J. Cancer 34, 1802–1806 (1998).
Kigawa, J. et al. Effect of p53 gene transfer and cisplatin in a peritonitis carcinomatosa model with p53-deficient ovarian cancer cells. Gynecol. Oncol. 84, 210–215 (2002).
Perego, P. et al. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 56, 556–562 (1996).
Pestell, K. E., Hobbs, S. M., Titley, J. C., Kelland, L. R. & Walton, M. I. Effect of p53 status on sensitivity to platinum complexes in a human ovarian cancer cell line. Mol. Pharmacol. 57, 503–511 (2000).
Fan, J. & Bertino, J. R. Modulation of cisplatinum cytotoxicity by p53: effect of p53-mediated apoptosis and DNA repair. Mol. Pharmacol. 56, 966–972 (1999).
Zamble, D. B., Jacks, T. & Lippard, S. J. p53-Dependent and-independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proc. Natl Acad. Sci. USA 95, 6163–6168 (1998).
Katayama, H. et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nature Genet. 36, 55–62 (2004).
Ohtsuka, T., Ryu, H., Minamishima, Y. A., Ryo, A. & Lee, S. W. Modulation of p53 and p73 levels by cyclin G: implication of a negative feedback regulation. Oncogene 22, 1678–1687 (2003).
Ohtsuka, T., Jensen, M. R., Kim, H. G., Kim, K. T. & Lee, S. W. The negative role of cyclin G in ATM-dependent p53 activation. Oncogene 23, 5405–5408 (2004).
Quinn, J. E. et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 63, 6221–6228 (2003).
Gatei, M. et al. Role for ATM in DNA damage-induced phosphorylation of BRCA1. Cancer Res. 60, 3299–3304 (2000).
Husain, A., He, G., Venkatraman, E. S. & Spriggs, D. R. BRCA1 upregulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res. 58, 1120–1123 (1998).
Hartman, A. R. & Ford, J. M. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nature Genet. 32, 180–184 (2002).
Olson, J. M. & Hallahan, A. R. p38 MAP kinase: a convergence point in cancer therapy. Trends. Mol. Med. 10, 125–129 (2004).
Wada, T. & Penninger, J. M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849 (2004).
Losa, J. H. et al. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene 22, 3998–4006 (2003). This paper describes that p38 MAPK pathway is a specific target for cisplatin-based therapy.
Kumar, S., Boehm, J. & Lee, J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature Rev. Drug Discovery 2, 717–726 (2003).
Pillaire, M. -J., Nebreda, A. R. & Darbon, J. -M. Cisplatin and UV radiation induce activation of the stress-activated protein kinase p38γ in human melanoma cells. Biochem. Biophys. Res. Comm. 278, 724–728 (2000).
Mansouri, A. et al. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J. Biol. Chem. 278, 19245–19256 (2003).
Yang, S. -H., Sharrocks, A. D. & Whitmarsh, A. J. Transcriptional regulation by the MAP kinase signaling cascades. Gene 320, 3–21 (2003).
Soloaga, A. et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 22, 2788–2797 (2003).
Thomson, S. et al. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 18, 4779–4793 (1999).
Wang, X., Martindale, J. L. & Holbrook, N. J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 275, 39435–39443 (2000).
Persons, D. L., Yazlovitskaya, E. M. & Pelling, J. C. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J. Biol. Chem. 275, 35778–35785 (2000).
Cui, W., Yazlovitskaya, E. M., Mayo, M. S., Pellings, J. C. & Persons, E. L. Cisplatin-induced response of c-jun N-terminal kinase 1 and extracellular signal-regulated protein kinases 1 and 2 in a series of cisplatin-resistant ovarian carcinoma cell lines. Mol. Carcinogenesis 29, 219–228 (2000).
Sánchez-Pérez, I., Murguía, J. R. & Perona, R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 16, 533–540 (1998).
Persons, D. L., Yazlovitskaya, E. M., Cui, W. & Pelling, J. C. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin. Cancer Res. 5, 1007–1014 (1999).
Hayakawa, J. et al. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J. Biol. Chem. 274, 31648–31654 (1999).
Mandic, A., Viktorsson, K., Heiden, T., Hansson, J. & Shoshan, M. C. The MEK1 inhibitor PD98059 sensitizes C8161 melanoma cells to cisplatin-induced apoptosis. Melanoma Res. 11, 11–19 (2001).
Yeh, P. Y. et al. Increase of the resistance of human cervical carcinoma cells to cisplatin by inhibition of the MEK to ERK signaling pathway partly via enhancement of anticancer drug-induced NF-κB activation. Biochem. Pharmacol. 63, 1423–1430 (2002).
Carter, A. B. & Hunninghake, G. W. A constitutive active MEK->ERK pathway negatively regulates NF-κB-dependent gene expression by modulating TATA-binding protein phosphorylation. J. Biol. Chem. 275, 27858–27864 (2000).
Gao, X. -S. et al. Sensitivity of anticancer drugs in NIH3T3' cells transfected with oncogenes accompanied by pSV2neo vector. Anticancer Res. 15, 1911–1914 (1995).
Zanke, B. W. et al. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol. 6, 606–613 (1996).
Sánchez-Pérez, I. & Perona, R. Lack of c-Jun activity increases survival to cisplatin. FEBS Lett. 453, 151–158 (1999).
Davis, R. J. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 (2000).
Levresse, V., Marek, L., Blumberg, D. & Heasley, L. E. Regulation of platinum-compound cytotoxicity by the c-Jun N-terminal kinase and c-Jun signaling pathway in small-cell lung cancer cells. Mol. Pharmacol. 62, 689–697 (2002).
Potapova, O. et al. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J. Biol. Chem. 272, 14041–14044 (1997).
Chen, Y. -R., Wang, X., Templeton, D., Davis, R. J. & Tan, T. -H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271, 31929–31936 (1996).
Sánchez-Pérez, I., Martínez-Gomariz, M., Williams, D., Keyse, S. M. & Perona, R. CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene 19, 5142–5152 (2000).
Franklin, C. C., Srikanth, S. & Kraft, A. S. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc. Natl Acad. Sci. USA 95, 3014–3019 (1998).
Dasika, G. K. et al. DNA damage-induced cell cycle checkpoints and DNA strand break repair in development and tumorigenesis. Oncogene 18, 7883–7899 (1999).
Attardi, L. D., de Vries, A. & Jacks, T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene 23, 973–980 (2004).
Sorenson, C. M. & Eastman, A. Influence of cis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excision repair proficient and deficient Chinese hamster ovary cells. Cancer Res. 48, 6703–6707 (1988).
Peng, C. -Y. et al. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501–1505 (1997).
Sanchez, Y. et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–1501 (1997).
Furnari, B., Rhind, N. & Russell, P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277, 1495–1497 (1997).
Liu, Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000).
Matsuoka, S., Huang, M. & Elledge, S. J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893–1897 (1998).
Matsuoka, S. et al. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl Acad. Sci. USA 97, 10389–10394 (2000).
Lopez-Girona, A., Furnari, B., Mondesert, O. & Russell, P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397, 172–175 (1999).
Costa, R. -M., Chigancas, V., Galhardo Rda, S. Carvalho, H. & Menck, C. F. The eukaryotic nucleotide excision repair pathway. Biochimie. 85, 1083–1099 (2003). A recent review about mechanisms of nucleotide excision repair (NER). This article describes the main protein players and the different sequential steps in the eukaryotic NER mechanism in human cells, from lesion recognition to damage removal and DNA synthesis.
Bohr, V. A., Smith, C. A., Okumoto, D. S. & Hanawalt, P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell 40, 359–369 (1985). This paper reports transcription coupled repair — a subpathway of nucleotide excision repair.
Mellon, I., Spivak, G. & Hanawalt, P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 51, 241–249 (1987).
Sugasawa, K. et al. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell 2, 223–232 (1998).
Spivak, G. et al. Ultraviolet-sensitive syndrome cells are defective in transcription-coupled repair of cyclobutane pyrimidine dimers. DNA Repair 1, 629–643 (2002).
Hanawalt, P. C. Subpathways of nucleotide excision repair and their regulation. Oncogene 21, 8949–8956 (2002).
Wang, D., Hara, R., Singh, G., Sancar, A. & Lippard, S. J. Nucleotide excision repair from site-specifically platinum-modified nucleosomes. Biochemistry 42, 6747–6753 (2003). This paper reveals that mononucleosome and post-translational modifications of histones modulate the excision repair of platinum–DNA adducts.
Furuta, T. et al. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 62, 4899–4902 (2002).
Wang, Z., Wu, X. & Friedberg, E. C. Nucleotide-excision repair of DNA in cell-free extracts of the yeast Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 90, 4907–4911 (1993).
Welsh, C. et al. Reduced levels of XPA, ERCC1 and XPF DNA repair proteins in testis tumor cell lines. Int. J. Cancer 110, 352–361 (2004).
Koberle, B., Masters, J. R., Hartley, J. A. & Wood, R. D. Defective repair of cisplatin-induced DNA damage caused by reduced XPA protein in testicular germ cell tumours. Curr. Biol. 9, 273–276 (1999).
Ford, J. M. & Hanawalt, P. C. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc. Natl Acad. Sci. USA 92, 8876–8880 (1995).
Hwang, B. J., Ford, J. M., Hanawalt, P. C. & Chu, G. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc. Natl Acad. Sci. USA 96, 424–428 (1999).
Takimoto, R. et al. BRCA1 transcriptionally regulates damaged DNA binding protein (DDB2) in the DNA repair response following UV-irradiation. Cancer Biol. Ther. 1, 177–186 (2002).
Smith, M. L. et al. p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol. Cell. Biol. 20, 3705–3714 (2000). This paper describes evidence suggesting a role for Gadd45 in DNA repair and in chromatin remodelling of templates concurrent with DNA repair, thus suggesting that Gadd45 might participate in the coupling between chromatin assembly and DNA repair.
Smith, M. L. et al. Antisense GADD45 expression results in decreased DNA repair and sensitizes cells to u. v.-irradiation or cisplatin. Oncogene 13, 2255–2263 (1996).
Bellacosa, A. Functional interactions and signaling properties of mammalian DNA mismatch repair proteins. Cell Death Differ. 8, 1076–1092 (2001).
Nehmé, A. et al. Differential induction of c-Jun NH2-terminal kinase and c-Abl kinase in DNA mismatch repair-proficient and-deficient cells exposed to cisplatin. Cancer Res. 57, 3253–3257 (1997).
Toft, N. J. et al. Msh2 status modulates both apoptosis and mutation frequency in the murine small intestine. Proc. Natl Acad. Sci. USA 96, 3911–3915 (1999).
Lage, H. & Dietel, M. Involvement of the DNA mismatch repair system in antineoplastic drug resistance. J. Cancer Res. Clin. Oncol. 125, 156–165 (1999).
Strathdee, G., MacKean, M. J., Illand, M. & Brown, R. A role for methylation of the hMLH1 promoter in loss of hMLH1 expression and drug resistance in ovarian cancer. Oncogene 18, 2335–2341 (1999). This paper indicates that methylation of the hMLH1 promoter might be a common mechanism for loss of hMLH1 expression and possibly for cisplatin-resistance in ovarian cancer.
Drummond, J. T., Anthoney, A., Brown, R. & Modrich, P. Cisplatin and adriamycin resistance are associated with MutLalpha and mismatch repair deficiency in an ovarian tumor cell line. J. Biol. Chem. 271, 19645–19648 (1996).
Sansom, O. J., Toft, N. J., Winton, D. J. & Clarke, A. R. Msh-2 suppresses in vivo mutation in a gene dose and lesion dependent manner. Oncogene 20, 3580–3584 (2001).
de las Alas, M. M., Aebi, S., Fink, D., Howell, S. B. & Los, G. Loss of DNA mismatch repair: effects on the rate of mutation to drug resistance. J. Natl Cancer Inst. 89, 1537–1541 (1997).
Samimi, G. et al. Analysis of MLH1 and MSH2 expression in ovarian cancer before and after platinum drug-based chemotherapy. Clin. Cancer Res. 6, 1415–1421 (2000).
Claij, N. & te Riele, H. Msh2 deficiency does not contribute to cisplatin resistance in mouse embryonic stem cells. Oncogene 23, 260–266 (2004).
Branch, P., Masson, M., Aquilina, G., Bignami, M. & Karran, P. Spontaneous development of drug resistance: mismatch repair and p53 defects in resistance to cisplatin in human tumor cells. Oncogene 19, 3138–3145 (2000).
Massey, A., Offman, J., Macpherson, P. & Karran, P. DNA mismatch repair and acquired cisplatin resistance in E. coli and human ovarian carcinoma cells. DNA Repair 2, 73–89 (2003).
Gonzalez, V. M., Fuertes, M. A., Alonso, C. & Perez, J. M. Is cisplatin-induced cell death always produced by apoptosis? Mol. Pharmacol. 59, 657–663 (2001).
Lieberthal, W., Triaca, V. & Levine, J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am. J. Physiol. 270, 700–708 (1996).
Nguewa, P. A., Fuertes, M. A., Alonso, C. & Peréz, J. M. Pharmacological modulation of Poly(ADP-ribose) polymerase-mediated cell death: exploitation in cancer chemotherapy. Mol. Pharmacol. 64, 1007–1014 (2003).
Herceg, Z. & Wang, Z. Q. Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat. Res. 477, 97–110 (2001). PARP and poly(ADP-ribosyl)ation are proposed to be important for the regulation of many cellular processes such as DNA repair, cell death, chromatin functions and genomic stability. This review summarizes the functions of PARP in these processes.
Fulda, S., Los, M., Friesen, C. & Debatin, K. -M. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. Int. J. Cancer 76, 105–114 (1998).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Kojima, H. et al. Abrogation of mitochondrial cytochrome C release and caspase-3 activation in acquired multidrug resistance. J. Biol. Chem. 273, 16647–16650 (1998).
Seki, K. et al. Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8,-3 and-6 in osteosarcoma. Cancer Chemother. Pharmacol. 45, 199–206 (2000).
Srinivasula, S. M., Ahmad, M., Fernades-Alnemri, T. & Alnemri, E. S. Autoactivation of precaspase-9 by Apaf-1 mediated oligomerization. Mol. Cell 1, 949–957 (1998).
Blanc, C. et al. Caspase-3 Is Essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res. 60, 4386–4390 (2000).
Henkels, K. M. & Turchi, J. J. Cisplatin-induced apoptosis proceeds by caspase-3-dependent and-independent pathways in cisplatin-resistant and-sensitive human ovarian cancer cell lines. Cancer Res. 59, 3077–3083 (1999).
Cummings, B. S. & Schnellmann, R. G. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and-independent pathways. J. Pharmacol. Exp. Ther. 302, 8–17 (2002).
Galea, A. M. & Murray, V. The interaction of cisplatin and analogues with DNA in reconstituted chromatin. Biochim. Biophys. Acta 1579, 142–152 (2002).
Millard, J. T. & Wilkes, E. E. cis- and trans-diamminedichloroplatinum(II) interstrand crosslinking of a defined sequence nucleosomal core particle. Biochemistry 39, 16046–16055 (2000).
Foka, M. & Paoletti, J. Interaction of cis-diamminedichloroplatinum (II) to chromatin. Specificity of the drug distribution. Biochem. Pharmacol. 35, 3283–3291 (1986).
Lippard, S. J. & Hoeschele, J. D. Binding of cis- and trans-dichlorodiammineplatinum(II) to the nucleosome core. Proc. Natl Acad. Sci. USA 76, 6091–6095 (1979).
Ciccarelli, R. B., Solomon, M. J., Varshavsky, A. & Lippard, S. J. In vivo effects of cis- and trans-diamminedichloroplatinum(II) on SV40 chromosomes: differential repair, DNA–protein crosslinking, and inhibition of replication. Biochemistry 24, 7533–7540 (1985).
Hayes, J. J. & Scovell, W. M. cis-diamminedichloroplatinum (II) modified chromatin and nucleosomal core particle. Biochim. Biophys. Acta 1089, 377–385 (1991).
Hayes, J. J. & Scovell, W. M. cis-diamminedichloroplatinum (II) modified chromatin and nucleosomal core particle probed with DNase I. Biochim. Biophys. Acta 1088, 413–418 (1991).
Mymryk, J. S., Zaniewski, E. & Archer, T. K. Cisplatin inhibits chromatin remodeling, transcription factor binding, and transcription from the mouse mammary tumor virus promoter in vivo. Proc. Natl Acad. Sci. USA 92, 2076–2080 (1995).
Ziegler, C. J., Silverman, A. P. & Lippard, S. J. High throughput synthesis and screening of platinum drug candidates. J. Biol. Inorg. Chem. 5, 774–783 (2000).
Giandomenico, C. M. et al. Carboxylation of kinetically inert platinum(IV) hydroxy complexes. An entree into orally active platinum(IV) antitumor agents. Inorg. Chem. 34, 1015–1021 (1995).
Hall, M. D., Dolman, R. C. & Hambley, T. W. in Metal Ions in Biological Systems (ed. Sigel, H.) 297–322 (Marcel Dekker, New York, 2004).
Pasqualini, R. et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 60, 722–727 (2000).
Arap, W., Pasqualini, R. & Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279, 377–380 (1998).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Jackson, A., Davis, J., Pither, R. J., Rodger, A. & Hannon, M. J. Estrogen-derived steroidal metal complexes: agents for cellular delivery of metal centers to estrogen receptor-positive cells. Inorg. Chem. 40, 3964–3973 (2001).
Bunn, P. A. Chemotherapy for advanced non-small-cell lung cancer: who, what, when, why? J. Clin. Oncol. 20, 23S–33S (2002).
Martin, M. Platinum compounds in the treatment of advanced breast cancer. Clin. Breast Cancer 2, 190–208 (2001).
Smith, I. E. & Talbot, D. C. Cisplatin and its analogues in the treatment of advanced breast cancer: a review. Br. J. Cancer 65, 787–793 (1992).
Cosaert, J. & Quoix, E. Platinum drugs in the treatment of non-small-cell lung cancer. Br. J. Cancer 87, 825–833 (2002).
Shelley, M. D., Burgon, K. & Mason, M. D. Treatment of testicular germ-cell cancer: a Cochrane evidence-based systematic review. Cancer Treat Rev 28, 237–253 (2002).
Lebwohl, D. & Canetta, R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur. J. Cancer 34, 1522–1534 (1998). This review covers the clinical development of platinum coordination complexes, with emphasis on those compounds still under active study.
de Mulder, P. H. The chemotherapy of head and neck cancer. Anticancer Drugs 10 (Suppl. 1), S33–S37 (1999).
Scanlon, K. J., Newman, E. M., Lu, Y. & Priest, D. G. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc. Natl Acad. Sci. USA 83, 8923–8925 (1986).
Scanlon, K. J., Lu, Y., Kashani-Sabet, M., Ma, J. & Newman, E. Mechanisms for cisplatin-FUra synergism and cisplatin resistance in human ovarian carcinoma cells both in vitro and in vivo. Adv. Exp. Med. Biol. 244, 127–135 (1988).
Shirasaka, T., Shimamoto, Y., Ohshimo, H., Saito, H. & Fukushima, M. Metabolic basis of the synergistic antitumor activities of 5-fluorouracil and cisplatin in rodent tumor models in vivo. Cancer Chemother. Pharmacol. 32, 167–172 (1993).
Johnston, P. G. et al. The cellular interaction of 5-fluorouracil and cisplatin in a human colon carcinoma cell line. Eur. J. Cancer 32A, 2148–2154 (1996).
Kim, M. S. et al. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 63, 7291–7300. (2003).
Bubley, G. J. et al. Effect of DNA conformation on cisplatin adduct formation. Biochem. Pharmacol. 51, 717–721 (1996).
Eastman, A. Crosslinking of glutathione to DNA by cancer chemotherapeutic platinum coordination complexes. Chem. Biol. Interact. 61, 241–248 (1987).
Hamilton, T. C. et al. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and-sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem. Pharmacol. 34, 2583–2586 (1985).
Lai, G. M., Ozols, R. F., Young, R. C. & Hamilton, T. C. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J. Natl Cancer Inst. 81, 535–539 (1989).
Richon, V. M., Schulte, N. & Eastman, A. Multiple mechanisms of resistance to cis-diamminedichloroplatinum(II) in murine leukemia L1210 cells. Cancer Res. 47, 2056–2061 (1987).
Kasahara, K. et al. Metallothionein content correlates with the sensitivity of human small cell lung cancer cell lines to cisplatin. Cancer Res. 51, 3237–3242 (1991).
Dabholkar, M., Vionnet, J., Bostick-Bruton, F., Yu, J. J. & Reed, E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J. Clin. Invest. 94, 703–708 (1994).
Lai, G. M., Ozols, R. F., Smyth, J. F., Young, R. C. & Hamilton, T. C. Enhanced DNA repair and resistance to cisplatin in human ovarian cancer. Biochem. Pharmacol. 37, 4597–4600 (1988).
Zeng-Rong, N. et al. Elevated DNA repair capacity is associated with intrinsic resistance of lung cancer to chemotherapy. Cancer Res. 55, 4760–4764 (1995).
Mamenta, E. L. et al. Enhanced replicative bypass of platinum–DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 54, 3500–3505 (1994).
Eliopoulos, A. G. et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene 11, 1217–1228 (1995).
Acknowledgements
This work was supported by a National Cancer Institute grant to S.J.L. We thank K. R. Barnes for a critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- V(D)J RECOMBINATION
-
Site-directed DNA recombination carried out by RAG1 and RAG2 to create an enormous diversity of immunoglobulins and T-cell receptors.
- NUCLEOTIDE EXCISION REPAIR
-
A DNA-repair pathway that removes ultraviolet-light-induced DNA damage and chemical DNA adducts by excising the oligonucleotide that contains the damaged base(s). The single-stranded gap is filled in by using the intact strand as a template.
- NUCLEOSOME
-
A packing unit for DNA within the cell nucleus, which gives the chromatin a 'beads-on-a-string' structure, in which the 'beads' consist of complexes of nuclear proteins (histones) and DNA, and the 'string' consists of DNA only. A histone octamer forms a core around which the double-stranded DNA helix is wound nearly twice.
- UBIQUITYLATION
-
A type of protein modification involving the covalent addition of ubiquitin, a small protein, to lysine groups. Poly-ubiquitylation targets proteins for degradation.
- CHECKPOINT
-
The cell cycle can be arrested before the G1–S or G2–M phase transitions at checkpoints where DNA damage is detected.
- COCKAYNE SYNDROME
-
A rare inherited disorder in which people are sensitive to sunlight, have short stature, and have the appearance of premature aging. Two genes defective in Cockayne syndrome, CSA and CSB, have been identified so far, both of which code for proteins that interact with components of the transcriptional machinery and with DNA repair proteins.
- MISMATCH REPAIR
-
A DNA-repair pathway that removes mismatched bases and corrects the insertion or deletion of short stretches of (repeated) DNA.
Rights and permissions
About this article
Cite this article
Wang, D., Lippard, S. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4, 307–320 (2005). https://doi.org/10.1038/nrd1691
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd1691
This article is cited by
-
Pharmacodynamics and biodistribution of [195mPt]cisplatin(CISSPECT®) in head and neck squamous cell carcinoma
EJNMMI Research (2024)
-
SH3RF2 contributes to cisplatin resistance in ovarian cancer cells by promoting RBPMS degradation
Communications Biology (2024)
-
Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance
Nature Cancer (2024)
-
Identification of TACSTD2 as novel therapeutic targets for cisplatin-induced acute kidney injury by multi-omics data integration
Human Genetics (2024)
-
Pd(II) and Pt(II) Mixed Ligand Complexes Containing 2,5-Dimercapto-1,3,4-Thiadizole and 1,2-Bis(diphenylphosphino)ethane Ligands, Synthesis, Characterization, Crystal Structure, Anticancer and Computational Studies
Chemistry Africa (2024)