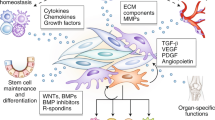
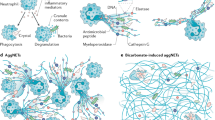
Abstract
The advent of in situ immunology and intravital analyses of leukocyte movement in tissues has drawn attention to the previously neglected extracellular matrix (ECM) and its role in modulating immune cell behaviour in inflamed tissues. The ECM exists in different biochemical and structural forms; both their individual components and three-dimensional ultrastructure impart specific signals to cells that modulate basic functions that are important for the early steps in inflammation, such as immune cell migration into inflamed tissues and immune cell differentiation. In chronically inflamed tissues, aberrant ECM expression and fragments of the ECM that are derived from tissue-remodelling processes can influence immune cell activation and survival, thereby actively contributing to immune responses at these sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nourshargh, S., Hordijk, P. L. & Sixt, M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nature Rev. Mol. Cell Biol. 11, 366–378 (2010). An up-to-date Review of cell–cell adhesion molecules, soluble factors and, in particular, junctional molecules that are involved in neutrophil extravasation, as well as an overview of leukocyte migration in the interstitial ECM.
Vestweber, D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol. Rev. 218, 178–196 (2007).
Alon, R. & Ley, K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr. Opin. Cell Biol. 20, 525–532 (2008).
Morrison, C. J., Butler, G. S., Rodriguez, D. & Overall, C. M. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr. Opin. Cell Biol. 21, 645–653 (2009).
Cauwe, B., Van den Steen, P. E. & Opdenakker, G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 42, 113–185 (2007).
Struyf, S., Proost, P. & Van Damme, J. Regulation of the immune response by the interaction of chemokines and proteases. Adv. Immunol. 81, 1–44 (2003).
Hohenester, E. & Engel, J. Domain structure and organisation in extracellular matrix proteins. Matrix Biol. 21, 115–128 (2002).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009). A comprehensive review of the domain structure of ECM molecules and their multiple functions.
Lokmic, Z. et al. The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Semin. Immunol. 20, 4–13 (2008).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Eckes, B., Nischt, R. & Krieg, T. Cell–matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 3, 4 (2010).
Engelhardt, B. & Wolburg, H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 34, 2955–2963 (2004).
Furie, M. B., Naprstek, B. L. & Silverstein, S. C. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J. Cell Sci. 88, 161–175 (1987).
Hurley, J. V. An electron microscopic study of leucocytic emigration and vascular permeability in rat skin. Aust. J. Exp. Biol. Med. Sci. 41, 171–186 (1963).
Marchesi, V. T. & Florey, H. W. Electron micrographic observations on the emigration of leucocytes. Q. J. Exp. Physiol. Cogn. Med. Sci. 45, 343–348 (1960).
Ohashi, K. L., Tung, D. K., Wilson, J., Zweifach, B. W. & Schmid-Schonbein, G. W. Transvascular and interstitial migration of neutrophils in rat mesentery. Microcirculation 3, 199–210 (1996).
Hoshi, O. & Ushiki, T. Neutrophil extravasation in rat mesenteric venules induced by the chemotactic peptide N-formyl-methionyl-luecylphenylalanine (fMLP), with special attention to a barrier function of the vascular basal lamina for neutrophil migration. Arch. Histol. Cytol. 67, 107–114 (2004).
Wang, S. et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 203, 1519–1532 (2006).
Bartholomaus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009). The only two-photon intravital microscopy analysis of T cell extravasation in vivo , showing cell retention at the vessel wall.
Muller, W. A. Mechanisms of transendothelial migration of leukocytes. Circ. Res. 105, 223–230 (2009).
Schenkel, A. R., Dufour, E. M., Chew, T. W., Sorg, E. & Muller, W. A. The murine CD99-related molecule CD99-like 2 (CD99L2) is an adhesion molecule involved in the inflammatory response. Cell Commun. Adhes. 14, 227–237 (2007).
Bixel, M. G. et al. A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood 109, 5327–5336 (2007).
Bixel, G. et al. CD99 and CD99L act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 116, 1172–1184 (2010).
Lou, O., Alcaide, P., Luscinskas, F. W. & Muller, W. A. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 178, 1136–1143 (2007).
Wakelin, M. W. et al. An anti-platelet-endothelial cell adhesion molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J. Exp. Med. 184, 229–239 (1996).
Dangerfield, J., Larbi, K. Y., Huang, M. T., Dewar, A. & Nourshargh, S. PECAM-1 (CD31) homophilic interaction up-regulates α6β1 on transmigrated neutrophils in vivo and plays a functional role in the ability of α6 integrins to mediate leukocyte migration through the perivascular basement membrane. J. Exp. Med. 196, 1201–1211 (2002).
Yurchenco, P. D. & Patton, B. L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 15, 1277–1294 (2009). An up-to-date overview of basement membrane components, their receptors and their functions.
Poschl, E. et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 (2004). The first study to show that basement membranes, including vascular basement membranes, can form and are stable without a type IV collagen network.
Li, S. et al. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157, 1279–1290 (2002).
McKee, K. K., Harrison, D., Capizzi, S. & Yurchenco, P. D. Role of laminin terminal globular domains in basement membrane assembly. J. Biol. Chem. 282, 21437–21447 (2007).
Koch, M. et al. A novel member of the netrin family, β-netrin, shares homology with the β chain of laminin: identification, expression, and functional characterization. J. Cell Biol. 151, 221–234 (2000).
Timpl, R., Sasaki, T., Kostka, G. & Chu, M. L. Fibulins: a versatile family of extracellular matrix proteins. Nature Rev. Mol. Cell Biol. 4, 479–489 (2003).
Brekken, R. A. & Sage, E. H. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 19, 569–580 (2000).
Sasaki, T. et al. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J. Mol. Biol. 301, 1179–1190 (2000).
Hallmann, R. et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 85, 979–1000 (2005).
Burns, A. R., Smith, C. W. & Walker, D. C. Unique structural features that influence neutrophil emigration into the lung. Physiol. Rev. 83, 309–336 (2003).
Sixt, M. et al. Endothelial cell laminin isoforms, laminin 8 and 10, play decisive roles in in T-cell recruitment across the blood-brain-barrier in an experimental autoimmune encephalitis model (EAE). J. Cell Biol. 153, 933–945 (2001).
Wu, C. et al. Endothelial basement membrane laminin α5 selectively inhibits T lymphocyte extravasation into the brain. Nature Med. 15, 519–527 (2009). This was the first study to show that laminin basement membrane composition selectively influences the extravasation of T cells.
Frieser, M. et al. Cloning of the mouse laminin α4 cDNA. Expression in a subset of endothelium. Eur. J. Biochem. 246, 727–735 (1997).
Khoshnoodi, J., Pedchenko, V. & Hudson, B. G. Mammalian collagen IV. Microsc. Res. Tech. 71, 357–370 (2008).
Iozzo, R. V. Basement membrane proteoglycans: from cellar to ceiling. Nature Rev. Mol. Cell Biol. 6, 646–656 (2005).
Bader, B. L. et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell Biol. 25, 6846–6856 (2005).
Sorokin, L. M. et al. Developmental regulation of laminin α5 suggests a role in epithelial and endothelial cell maturation. Dev. Biol. 189, 285–300 (1997).
Brachvogel, B. et al. Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Exp. Cell Res. 313, 2730–2743 (2007).
Stratman, A. N., Malotte, K. M., Mahan, R. D., Davis, M. J. & Davis, G. E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091–5101 (2009).
Abramsson, A. et al. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF–BB binding and pericyte recruitment in vascular development. Genes Dev. 21, 316–331 (2007).
Kalamajski, S. & Oldberg, A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 29, 248–253 (2010).
Rowe, R. G. & Weiss, S. J. Breaching the basement membrane: who, when and how? Trends Cell Biol. 18, 560–574 (2008). An overview of the potential mechanisms that are used by different cell types to penetrate basement membranes.
Senior, R. M., Gresham, H. D., Griffin, G. L., Brown, E. J. & Chung, A. E. Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin. J. Clin. Invest. 90, 2251–2257 (1992).
Adair-Kirk, T. L. et al. A site on laminin α5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J. Immunol. 171, 398–406 (2003).
Pipoly, D. J. & Crouch, E. C. Degradation of native type IV procollagen by human neutrophil elastase. Implications for leukocyte-mediated degradation of basement membranes. Biochemistry 26, 5748–5754 (1987).
Heck, L. W., Blackburn, W. D., Irwin, M. H. & Abrahamson, D. R. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am. J. Pathol. 136, 1267–1274 (1990).
Steadman, R. et al. Laminin cleavage by activated human neutrophils yields proteolytic fragments with selective migratory properties. J. Leukoc. Biol. 53, 354–365 (1993).
Huber, A. R. & Weiss, S. J. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J. Clin. Invest. 83, 1122–1136 (1989).
Agrawal, S. et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203, 1007–1019 (2006).
Huber, A. R., Kunkel, S. L., Todd, R. F. 3rd & Weiss, S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science 254, 99–102 (1991).
Marchesi, V. T. The site of leucocyte emigration during inflammation. Q. J. Exp. Physiol. Cogn. Med. Sci. 46, 115–118 (1961).
Steadman, R. et al. Human neutrophils do not degrade major basement membrane components during chemotactic migration. Int. J. Biochem. Cell Biol. 29, 993–1004 (1997).
Pham, C. T. Neutrophil serine proteases: specific regulators of inflammation. Nature Rev. Immunol. 6, 541–550 (2006).
Walker, D. C., Behzad, A. R. & Chu, F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc. Res. 50, 397–416 (1995).
Abrams, G. A., Goodman, S. L., Nealey, P. F., Franco, M. & Murphy, C. J. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 299, 39–46 (2000).
Candiello, J. et al. Biomechanical properties of native basement membranes. FEBS J. 274, 2897–2908 (2007).
Last, J. A., Liliensiek, S. J., Nealey, P. F. & Murphy, C. J. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J. Struct. Biol. 167, 19–24 (2009).
Kenne, E. et al. Immune cell recruitment to inflammatory loci is impaired in mice deficient in basement membrane protein laminin α4. J. Leukoc. Biol. 29 Apr 2010 (doi:10.1189/jlb.0110043).
Engelhardt, B. & Sorokin, L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 31, 497–511 (2009).
Wekerle, H. Lessons from multiple sclerosis: models, concepts, observations. Ann. Rheum. Dis. 67, 56–60 (2008).
Thyboll, J. et al. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol. Cell Biol. 22, 1194–1202 (2002).
Moore, S. A. et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418, 422–425 (2002).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008). The first study to show a non-integrin, non-proteolytic, force-dependent mode of DC migration through the interstitial matrix.
Wolf, K., Muller, R., Borgmann, S., Brocker, E. B. & Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3269–3269 (2003).
Friedl, P. & Wolf, K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem. Soc. Symp. 70, 277–285 (2003).
Sabeh, F., Li, X. Y., Saunders, T. L., Rowe, R. G. & Weiss, S. J. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J. Biol. Chem. 284, 23001–23011 (2009).
Sabeh, F., Shimizu-Hirota, R. & Weiss, S. J. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J. Cell Biol. 185, 11–19 (2009).
Rabodzey, A., Alcaide, P., Luscinskas, F. W. & Ladoux, B. Mechanical forces induced by the transendothelial migration of human neutrophils. Biophys. J. 95, 1428–1438 (2008).
Oakes, P. W. et al. Neutrophil morphology and migration are affected by substrate elasticity. Blood 114, 1387–1395 (2009).
Shulman, Z. et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 30, 384–396 (2009).
Voisin, M. B., Woodfin, A. & Nourshargh, S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler. Thromb. Vasc. Biol. 29, 1193–1199 (2009).
Weathington, N. M. et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nature Med. 12, 317–323 (2006). One of the few clear descriptions of how MMP processing of an interstitial matrix molecule, type I collagen, results in a fragment chemoattractant for neutrophils during lung inflammation.
Gaggar, A. et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 180, 5662–5669 (2008).
O'Reilly, P. J. et al. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J. Neuroimmunol. 217, 51–54 (2009).
Senior, R. M., Griffin, G. L. & Mecham, R. P. Chemotactic activity of elastin-derived peptides. J. Clin. Invest. 66, 859–862 (1980).
Hunninghake, G. W. et al. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science 212, 925–927 (1981).
Houghton, A. M. et al. Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 116, 753–759 (2006).
Ospelt, C. & Gay, S. TLRs and chronic inflammation. Int. J. Biochem. Cell Biol. 42, 495–505 (2010).
Oldberg, A., Franzen, A. & Heinegard, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc. Natl Acad. Sci. USA 83, 8819–8823 (1986).
Wong, C. K., Lit, L. C., Tam, L. S., Li, E. K. & Lam, C. W. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford) 44, 602–606 (2005).
Xu, G. et al. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J. Clin. Invest. 115, 1060–1067 (2005).
Sato, T. et al. Osteopontin/Eta-1 upregulated in Crohn's disease regulates the Th1 immune response. Gut 54, 1254–1262 (2005).
Chabas, D. et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294, 1731–1735 (2001).
Comabella, M. et al. Plasma osteopontin levels in multiple sclerosis. J. Neuroimmunol. 158, 231–239 (2005).
Hur, E. M. et al. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature Immunol. 8, 74–83 (2007). A description of the mechanism of action of the ECM molecule osteopontin in T H 1 and T H 17 cell polarization during EAE and in inhibition of effector T cell apoptosis.
Steinman, L. A molecular trio in relapse and remission in multiple sclerosis. Nature Rev. Immunol. 9, 440–447 (2009).
Ashkar, S. et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287, 860–864 (2000).
Adler, B., Ashkar, S., Cantor, H. & Weber, G. F. Costimulation by extracellular matrix proteins determines the response to TCR ligation. Cell. Immunol. 210, 30–40 (2001).
Pender, M. P., Nguyen, K. B., McCombe, P. A. & Kerr, J. F. Apoptosis in the nervous system in experimental allergic encephalomyelitis. J. Neurol. Sci. 104, 81–87 (1991).
Gold, R., Hartung, H. P. & Lassmann, H. T-cell apoptosis in autoimmune diseases: termination of inflammation in the nervous system and other sites with specialized immune-defense mechanisms. Trends Neurosci. 20, 399–404 (1997).
Hocking, A. M., Shinomura, T. & McQuillan, D. J. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 17, 1–19 (1998).
Okamura, Y. et al. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276, 10229–10233 (2001).
Stern, R., Asari, A. A. & Sugahara, K. N. Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 85, 699–715 (2006). A good overview of hyaluronan synthesis, fragment generation and studies on the role of hyaluronan in activating TLRs.
Johnson, G. B., Brunn, G. J., Kodaira, Y. & Platt, J. L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 168, 5233–5239 (2002).
Taylor, K. R. & Gallo, R. L. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 20, 9–22 (2006).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nature Med. 15, 774–780 (2009). A description of the activation of TLR2 and TLR4 by tenascin during rheumatoid arthritis.
Schaefer, L. et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 115, 2223–2233 (2005).
Schaefer, L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr. Opin. Pharmacol. 10, 185–190 (2010).
Jiang, D. et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Med. 11, 1173–1179 (2005).
Noble, P. W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 21, 25–29 (2002).
McKee, C. M. et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Invest. 98, 2403–2413 (1996).
Mummert, M. E. et al. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J. Immunol. 169, 4322–4331 (2002).
Termeer, C. et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 195, 99–111 (2002).
Overall, C. M. & Blobel, C. P. In search of partners: linking extracellular proteases to substrates. Nature Rev. Mol. Cell Biol. 8, 245–257 (2007). A Review of new concepts of MMPs action and current modes of substrate identification.
Vanacore, R. M. et al. The α1.α2 network of collagen IV. Reinforced stabilization of the noncollagenous domain-1 by noncovalent forces and the absence of Met-Lys cross-links. J. Biol. Chem. 279, 44723–44730 (2004).
Nagase, H., Visse, R. & Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573 (2006).
Di Girolamo, N. et al. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: role in cell migration. J. Immunol. 177, 2638–2650 (2006).
Qiu, Z. et al. Interleukin-17 regulates chemokine and gelatinase B expression in fibroblasts to recruit both neutrophils and monocytes. Immunobiology 214, 835–842 (2009).
Hu, J., Van den Steen, P. E., Sang, Q. X. & Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nature Rev. Drug Discov. 6, 480–498 (2007). An overview of the potential roles of MMPs in inflammation.
Overall, C. M., McQuibban, G. A. & Clark-Lewis, I. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: a new tool for degradomics. Biol. Chem. 383, 1059–1066 (2002).
Van den Steen, P. E., Proost, P., Wuyts, A., Van Damme, J. & Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood 96, 2673–2681 (2000).
McQuibban, G. A. et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289, 1202–1206 (2000).
Van Den Steen, P. E. et al. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 270, 3739–3749 (2003).
Gearing, A. J. et al. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 370, 555–557 (1994).
Ito, A. et al. Degradation of interleukin 1β by matrix metalloproteinases. J. Biol. Chem. 271, 14657–14660 (1996).
Schonbeck, U., Mach, F. & Libby, P. Generation of biologically active IL-1β by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1β processing. J. Immunol. 161, 3340–3346 (1998).
Redondo-Munoz, J. et al. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia B cell survival through its hemopexin domain. Cancer Cell 17, 160–172 (2010).
Denhardt, D. T., Noda, M., O'Regan, A. W., Pavlin, D. & Berman, J. S. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 107, 1055–1061 (2001).
Leask, A. & Abraham, D. J. TGF-β signaling and the fibrotic response. FASEB J. 18, 816–827 (2004).
Hu, X. & Ivashkiv, L. B. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009).
McQuibban, G. A. et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 276, 43503–43508 (2001).
McQuibban, G. A. et al. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 100, 1160–1167 (2002).
Hayashida, K., Parks, W. C. & Park, P. W. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood 114, 3033–3043 (2009).
Acknowledgements
I apologize to those individuals whose work I have not been able to cite owing to space limitations. I thank R. Hallmann, P. Bruckner, D. Vestweber, P. Yurchenco and V. Gerke for critical reading of the manuscript and for helpful suggestions. Special thanks go to C. Wu for help with figure 2. Some of the research that is described in this Review was supported by the German Research Foundation (DFG), the Swedish Research Council (VR), the Medical Faculty of the University of Münster, Germany, and the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreements 201,024 and 202,213 (European Stroke Network).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Intravital microscopy
-
A technique that is used for the examination of biological processes, such as leukocyte–endothelial cell interactions, in living tissues. In general, translucent tissues are used, such as the mesentery or cremaster muscle. These tissues can be exposed and mounted for microscopic observation.
- Extracellular matrix
-
(ECM). The secreted products of many cell types that form an organized scaffold for cell support.
- Extravasation
-
The cellular process in which circulating leukocytes bind to and migrate through the endothelium into the underlying tissue.
- Cell adhesion molecules
-
Proteins (for example, CD99, CD99 antigen-like-2 (CD99L2) and platelet/endothelial cell adhesion molecule 1 (PECAM1)) that are located between adjacent endothelial cells but that are also expressed by neutrophils, monocytes and lymphocytes and are known to have important roles in leukocyte transmigration of the endothelium in inflammation. CD99 (also known as MIC2) is a small highly glycosylated glycoprotein with no known similarity to any other protein family. CD99L2 is distantly related to CD99 and has 32% sequence identity; PECAM1 is a member of the immunoglobulin supergene family.
- Pericytes
-
Cells that are embedded in the vascular basement membrane of microvessels and that are thought to be derived from the vascular smooth muscle cell lineage. They make close cellular contact with endothelial cells and this interaction is essential for the maintenance of vessel function, as well as for the regulation of angiogenesis and vascular remodelling.
- Tensile strength
-
The maximum load that a material can support during stretching without irreversible disruption and is expressed per unit area. When stresses less than the tensile strength are removed, a material returns either completely or partially to its original shape and size.
- Diapedesis
-
The migration of leukocytes across the endothelium. This migration generally occurs by squeezing through the junctions between adjacent endothelial cells, although in some settings leukocytes have been shown to pass through transiently formed gaps in the cytoplasm of endothelial cells. It is the last step in the leukocyte–endothelial cell adhesion cascade, which includes tethering, triggering, tight adhesion and transmigration.
- Perivascular cuff
-
The immune cell infiltrate that is immediately adjacent to the outer surface of the post-capillary venule wall and, in the case of central nervous system vessels, is bordered by the inner endothelial cell basement membrane and the outer parenchymal basement membrane.
- Delayed-type hypersensitivity
-
(DTH). A cellular immune response to antigen that develops over a period of ∼24–72 hours. The response is characterized by the infiltration of T cells and monocytes and depends on the production of TH1-type cytokines.
- Filopodia
-
Slender cytoplasmic projections that extend from the leading edge of migrating cells.
- Osteopontin
-
An extracellular matrix protein that supports the adhesion and migration of inflammatory cells. It has recently been recognized as an immunoregulatory TH1-type cytokine.
- Toll-like receptor family
-
(TLR family). A family of receptors that are unique to microorganisms that recognize conserved products (such as lipopolysaccharide) known as pathogen-associated molecular patterns (PAMPs), as well as damage-associated molecular patterns (DAMPs). TLRs signal to the host that a microbial pathogen is present or that tissue damage has occurred.
Rights and permissions
About this article
Cite this article
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10, 712–723 (2010). https://doi.org/10.1038/nri2852
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2852