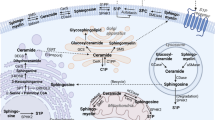
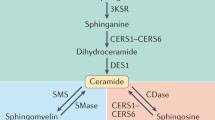
Key Points
-
Bioactive sphingolipids constitute a family of lipids, including sphingosine, ceramide, sphingosine-1-phosphate (S1P) and ceramide-1-phosphate. These molecules act on distinct protein targets, including kinases, phosphatases, lipases and other enzymes and membrane receptors, and they exert distinct cellular functions.
-
This universe of sphingolipids, the sphingolipidome, is highly complex, with distinct molecular species of each of the bioactive lipids and metabolic interconnections that interconvert one bioactive lipid into others (for example, ceramide to sphingosine and then to S1P). Critically, these pathways demonstrate specific subcellular localizations that appear to dictate the specific functions of sphingolipids.
-
A plethora of cell biological processes are critically modulated by bioactive sphingolipids, including growth regulation, cell migration, adhesion, apoptosis, senescence and inflammatory responses.
-
At the tissue and organismal level, bioactive sphingolipids have been implicated in neurodegenerative processes, metabolic disorders, various cancers (and various cancer attributes), immune function, cardiovascular disorders and skin integrity.
-
Major advances have been made in defining the enzymes of sphingolipid metabolism, their mechanisms and their structures. However, a major challenge is to decipher the biochemical mechanisms by which these enzymes and their products are specifically regulated.
-
A key future challenge is to determine the molecular mechanisms of action for specific species or subgroups of bioactive sphingolipids (for example, distinct ceramides and distinct sphingoid bases).
Abstract
Studies of bioactive lipids in general and sphingolipids in particular have intensified over the past several years, revealing an unprecedented and unanticipated complexity of the lipidome and its many functions, which rivals, if not exceeds, that of the genome or proteome. These results highlight critical roles for bioactive sphingolipids in most, if not all, major cell biological responses, including all major cell signalling pathways, and they link sphingolipid metabolism to key human diseases. Nevertheless, the fairly nascent field of bioactive sphingolipids still faces challenges in its biochemical and molecular underpinnings, including defining the molecular mechanisms of pathway and enzyme regulation, the study of lipid–protein interactions and the development of cellular probes, suitable biomarkers and therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 (2008).
Thudichum, J. L. W. A Treatise on the Chemical Constitution of the Brain (Archon Books, 1962). This is the first documented isolation of the sphingolipids, and includes the coining of the term 'sphingosin'.
Hannun, Y. A. & Obeid, L. M. Many ceramides. J. Biol. Chem. 286, 27855–27862 (2011). This review advances the hypothesis that ceramides are indeed a family of distinct molecular species that are products of distinct metabolic enzymes and that the different ceramides may have distinct functions.
Schulze, H. & Sandhoff, K. Sphingolipids and lysosomal pathologies. Biochim. Biophys. Acta 1841, 799–810 (2014).
Huang, X., Withers, B. R. & Dickson, R. C. Sphingolipids and lifespan regulation. Biochim. Biophys. Acta 1841, 657–664 (2014).
Astudillo, L. et al. Human genetic disorders of sphingolipid biosynthesis. J. Inherit. Metab. Dis. 38, 65–76 (2015). This is a comprehensive presentation of the various genetic disorders that are directly caused by defects in sphingolipid metabolism.
Bode, H. et al. HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum. Mol. Genet. 25, 853–865 (2016).
Hornemann, T. et al. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 284, 26322–26330 (2009).
Harmon, J. M. et al. Topological and functional characterization of the ssSPTs, small activating subunits of serine palmitoyltransferase. J. Biol. Chem. 288, 10144–10153 (2013).
Cingolani, F., Futerman, A. H. & Casas, J. Ceramide synthases in biomedical research. Chem. Phys. Lipids 197, 25–32 (2016).
Wegner, M. S., Schiffmann, S., Parnham, M. J., Geisslinger, G. & Grosch, S. The enigma of ceramide synthase regulation in mammalian cells. Prog. Lipid Res. 63, 93–119 (2016). This is a comprehensive presentation of the functions and regulation of the family of CerSs.
Sassa, T. & Kihara, A. Metabolism of very long-chain Fatty acids: genes and pathophysiology. Biomol. Ther. 22, 83–92 (2014).
Grond, S. et al. PNPLA1 deficiency in mice and humans leads to a defect in the synthesis of omega-O-acylceramides. J. Invest. Dermatol. 137, 394–402 (2017).
Senkal, C. E. et al. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 25, 686–697 (2017). This study describes a novel pathway by which ceramide can be diverted or stored as O-acyl-ceramide in lipid droplets.
Ferreira, N. S. et al. Regulation of very-long acyl chain ceramide synthesis by acyl-CoA binding protein. J. Biol. Chem. 292, 7588–7597 (2017).
Wakashima, T., Abe, K. & Kihara, A. Dual functions of the trans-2-enoyl-CoA reductase TER in the sphingosine 1-phosphate metabolic pathway and in fatty acid elongation. J. Biol. Chem. 289, 24736–24748 (2014). This study identifies a key enzyme involved in the metabolism and recycling of fatty aldehydes after their generation from the breakdown of S1P.
Cabukusta, B. et al. ER residency of the ceramide phosphoethanolamine synthase SMSr relies on homotypic oligomerization mediated by its SAM domain. Sci. Rep. 7, 41290 (2017).
Rajagopalan, V. et al. Critical determinants of mitochondria-associated neutral sphingomyelinase (MA-nSMase) for mitochondrial localization. Biochim. Biophys. Acta 1850, 628–639 (2015).
Murate, M. et al. Transbilayer distribution of lipids at nano scale. J. Cell Sci. 128, 1627–1638 (2015).
Abe, M. & Kobayashi, T. Imaging local sphingomyelin-rich domains in the plasma membrane using specific probes and advanced microscopy. Biochim. Biophys. Acta 1841, 720–726 (2014).
Deng, Y., Rivera-Molina, F. E., Toomre, D. K. & Burd, C. G. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl Acad. Sci. USA 113, 6677–6682 (2016).
Nagahashi, M. et al. Sphingosine-1-phosphate transporters as targets for cancer therapy. BioMed Res. Int. 2014, 651727 (2014).
Wadsworth, J. M. et al. The chemical basis of serine palmitoyltransferase inhibition by myriocin. J. Am. Chem. Soc. 135, 14276–14285 (2013).
Zhou, Y. F. et al. Human acid sphingomyelinase structures provide insight to molecular basis of Niemann-Pick disease. Nat. Commun. 7, 13082 (2016).
Gorelik, A., Illes, K., Heinz, L. X., Superti-Furga, G. & Nagar, B. Crystal structure of mammalian acid sphingomyelinase. Nat. Commun. 7, 12196 (2016).
Xiong, Z. J., Huang, J., Poda, G., Pomes, R. & Prive, G. G. Structure of human acid sphingomyelinase reveals the role of the saposin domain in activating substrate hydrolysis. J. Mol. Biol. 428, 3026–3042 (2016).
Gorelik, A., Liu, F., Illes, K. & Nagar, B. Crystal structure of the human alkaline sphingomyelinase provides insights into substrate recognition. J. Biol. Chem. 292, 7087–7094 (2017).
Dvir, H. et al. X-Ray structure of human acid-β-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 4, 704–709 (2003).
Wang, Z. et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure 21, 798–809 (2013).
Airola, M. V. et al. Structural basis for ceramide recognition and hydrolysis by human neutral ceramidase. Structure 23, 1482–1491 (2015).
Zhu, G., Koszelak-Rosenblum, M., Connelly, S. M., Dumont, M. E. & Malkowski, M. G. The crystal structure of an integral membrane fatty acid α-hydroxylase. J. Biol. Chem. 290, 29820–29833 (2015).
Vasiliauskaite-Brooks, I. et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017).
Hanson, M. A. et al. Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 (2012). This study describes the crystal structure of S1PR1.
Kudo, N. et al. Crystal structures of the CERT START domain with inhibitors provide insights into the mechanism of ceramide transfer. J. Mol. Biol. 396, 245–251 (2010).
Simanshu, D. K. et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 500, 463–467 (2013). This study describes the crystal structure of the C1P transporter.
Samygina, V. R. et al. Enhanced selectivity for sulfatide by engineered human glycolipid transfer protein. Structure 19, 1644–1654 (2011).
Sanchez, T. & Hla, T. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 92, 913–922 (2004).
Hait, N. C. et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325, 1254–1257 (2009). This study identifies HDACs as direct nuclear targets of S1P.
Galadari, S., Rahman, A., Pallichankandy, S. & Thayyullathil, F. Tumor suppressive functions of ceramide: evidence and mechanisms. Apoptosis 20, 689–711 (2015).
Mehra, V. C. et al. Ceramide-activated phosphatase mediates fatty acid-induced endothelial VEGF resistance and impaired angiogenesis. Am. J. Pathol. 184, 1562–1576 (2014).
Apostolidis, S. A. et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 17, 556–564 (2016).
Teixeira, V. & Costa, V. Unraveling the role of the target of rapamycin signaling in sphingolipid metabolism. Prog. Lipid Res. 61, 109–133 (2016). This is a comprehensive review of sphingolipid metabolism, function and regulation in yeast.
Taniguchi, M. et al. Lysosomal ceramide generated by acid sphingomyelinase triggers cytosolic cathepsin B-mediated degradation of X-linked inhibitor of apoptosis protein in natural killer/T lymphoma cell apoptosis. Cell Death Dis. 6, e1717 (2015).
Jain, A., Beutel, O., Ebell, K., Korneev, S. & Holthuis, J. C. Diverting CERT-mediated ceramide transport to mitochondria triggers Bax-dependent apoptosis. J. Cell Sci. 130, 360–371 (2017).
Birbes, H. et al. A mitochondrial pool of sphingomyelin is involved in TNFα-induced Bax translocation to mitochondria. Biochem. J. 386, 445–451 (2005).
Spiegel, S. & Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 (2011).
Chaurasia, B. & Summers, S. A. Ceramides — lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Montefusco, D. J., Matmati, N. & Hannun, Y. A. The yeast sphingolipid signaling landscape. Chem. Phys. Lipids 177, 26–40 (2014).
Epstein, S. & Riezman, H. Sphingolipid signaling in yeast: potential implications for understanding disease. Front. Biosci. 5, 97–108 (2013).
Matmati, N. et al. Identification of C18:1-phytoceramide as the candidate lipid mediator for hydroxyurea resistance in yeast. J. Biol. Chem. 288, 17272–17284 (2013).
Chauhan, N., Visram, M., Cristobal-Sarramian, A., Sarkleti, F. & Kohlwein, S. D. Morphogenesis checkpoint kinase Swe1 is the executor of lipolysis-dependent cell-cycle progression. Proc. Natl Acad. Sci. USA 112, E1077–E1085 (2015).
Adada, M. M. et al. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 29, 4654–4669 (2015).
Bretscher, A., Edwards, K. & Fehon, R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 (2002).
van der Weyden, L. et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 541, 233–236 (2017). In an unbiased screen, this study identifies SPNS2, the S1P transporter, as a key regulator of metastasis.
Romero-Guevara, R., Cencetti, F., Donati, C. & Bruni, P. Sphingosine 1-phosphate signaling pathway in inner ear biology. New therapeutic strategies for hearing loss? Front. Aging Neurosci. 7, 60 (2015).
Kitajiri, S. et al. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J. Cell Biol. 166, 559–570 (2004).
Chen, J. et al. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS Genet. 10, e1004688 (2014).
Canals, D., Roddy, P. & Hannun, Y. A. Protein phosphatase 1α mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. J. Biol. Chem. 287, 10145–10155 (2012).
Carreira, A. C., Ventura, A. E., Varela, A. R. & Silva, L. C. Tackling the biophysical properties of sphingolipids to decipher their biological roles. Biol. Chem. 396, 597–609 (2015).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 (2008). This study ascribes a key role for ceramide and for nSMase2 in the regulation of exocytosis.
Kosaka, N. et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285, 17442–17452 (2010).
Guo, B. B., Bellingham, S. A. & Hill, A. F. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J. Biol. Chem. 290, 3455–3467 (2015).
Yuyama, K., Sun, H., Mitsutake, S. & Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J. Biol. Chem. 287, 10977–10989 (2012).
Shen, H. et al. Coupling between endocytosis and sphingosine kinase 1 recruitment. Nat. Cell Biol. 16, 652–662 (2014).
Hayashi, Y. et al. Sphingomyelin synthase 2, but not sphingomyelin synthase 1, is involved in HIV-1 envelope-mediated membrane fusion. J. Biol. Chem. 289, 30842–30856 (2014).
Contreras, F. X. et al. Molecular recognition of a single sphingolipid species by a protein's transmembrane domain. Nature 481, 525–529 (2012). This study identifies a specific molecular species of sphingomyelin, C18 sphingomyelin, as a ligand for p24, a component of the COPI secretion machinery.
Capasso, S. et al. Sphingolipid metabolic flow controls phosphoinositide turnover at the trans-Golgi network. EMBO J. 36, 1736–1754 (2017).
Heffernan-Stroud, L. A. et al. Defining a role for sphingosine kinase 1 in p53-dependent tumors. Oncogene 31, 1166–1175 (2012).
Wang, Y. et al. Alkaline ceramidase 2 is a novel direct target of p53 and induces autophagy and apoptosis through ROS generation. Sci. Rep. 7, 44573 (2017).
Shamseddine, A. A. et al. P53-dependent upregulation of neutral sphingomyelinase-2: role in doxorubicin-induced growth arrest. Cell Death Dis. 6, e1947 (2015).
Guillas, I. et al. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J. 20, 2655–2665 (2001). This study identifies the genes encoding CerSs ( lag1 and lac1 ) in yeast and demonstrates that these genes are in fact the first genes to be implicated in regulation of yeast lifespan.
Mosbech, M. B. et al. Functional loss of two ceramide synthases elicits autophagy-dependent lifespan extension in C. elegans. PLoS ONE 8, e70087 (2013).
Jazwinski, S. M. et al. HRAS1 and LASS1 with APOE are associated with human longevity and healthy aging. Aging Cell 9, 698–708 (2010).
Dany, M. & Ogretmen, B. Ceramide induced mitophagy and tumor suppression. Biochim. Biophys. Acta 1853, 2834–2845 (2015).
Siddique, M. M., Li, Y., Chaurasia, B., Kaddai, V. A. & Summers, S. A. Dihydroceramides: from bit players to lead actors. J. Biol. Chem. 290, 15371–15379 (2015). This is an informative summary of the roles of ceramides and dihydroceramides in metabolic pathways.
Hernandez-Tiedra, S. et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 12, 2213–2229 (2016).
Obeid, L. M., Linardic, C. M., Karolak, L. A. & Hannun, Y. A. Programmed cell death induced by ceramide. Science 259, 1769–1771 (1993).
Siskind, L. J. et al. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J. Biol. Chem. 285, 11818–11826 (2010).
Brinkmann, V. & Lynch, K. R. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr. Opin. Immunol. 14, 569–575 (2002).
Benechet, A. P. et al. T cell-intrinsic S1PR1 regulates endogenous effector T-cell egress dynamics from lymph nodes during infection. Proc. Natl Acad. Sci. USA 113, 2182–2187 (2016).
Hla, T., Venkataraman, K. & Michaud, J. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta 1781, 477–482 (2008).
Allende, M. L., Dreier, J. L., Mandala, S. & Proia, R. L. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401 (2004).
Breart, B. et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208, 1267–1278 (2011).
Blaho, V. A. et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523, 342–346 (2015). This study demonstrates specific immune functions for HDL-bound S1P in the circulation.
Pettus, B. J. et al. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol. Pharmacol. 68, 330–335 (2005).
Xiong, Y. et al. Sphingosine kinases are not required for inflammatory responses in macrophages. J. Biol. Chem. 291, 11465 (2016).
Jenkins, R. W. et al. Regulation of CC ligand 5/RANTES by acid sphingomyelinase and acid ceramidase. J. Biol. Chem. 286, 13292–13303 (2011).
Kott, M. et al. Acid sphingomyelinase serum activity predicts mortality in intensive care unit patients after systemic inflammation: a prospective cohort study. PLoS ONE 9, e112323 (2014).
Hannun, Y. A., Luberto, C., Mao, C. & Obeid, L. M. Bioactive Sphingolipids in Cancer Biology and Therapy (Springer, 2015).
Morad, S. A. & Cabot, M. C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 13, 51–65 (2013).
Pettus, B. J. et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-α. FASEB J. 17, 1411–1421 (2003).
Tan, S. S. et al. Sphingosine kinase 1 promotes malignant progression in colon cancer and independently predicts survival of patients with colon cancer by competing risk approach in South asian population. Clin. Transl Gastroenterol. 5, e51 (2014).
Kawamori, T. et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 23, 405–414 (2009).
Liang, J. et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 23, 107–120 (2013).
Kohno, M. et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 26, 7211–7223 (2006).
Oskouian, B. et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl Acad. Sci. USA 103, 17384–17389 (2006).
Ju, T., Gao, D. & Fang, Z. Y. Targeting colorectal cancer cells by a novel sphingosine kinase 1 inhibitor PF-543. Biochem. Biophys. Res. Commun. 470, 728–734 (2016).
Chumanevich, A. A. et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 31, 1787–1793 (2010).
García-Barros, M. et al. Role of neutral ceramidase in colon cancer. FASEB J. 30, 4159–4171 (2016).
Heffernan-Stroud, L. A. & Obeid, L. M. Sphingosine kinase 1 in cancer. Adv. Cancer Res. 117, 201–235 (2013).
Galvani, S. et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 8, ra79 (2015).
Nagahashi, M. et al. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv. Biol. Regul. 54, 112–120 (2014).
Anelli, V., Gault, C. R., Snider, A. J. & Obeid, L. M. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 24, 2727–2738 (2010).
Mahdy, A. E. et al. Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol. Ther. 17, 430–438 (2009).
Frohbergh, M., He, X. & Schuchman, E. H. The molecular medicine of acid ceramidase. Biol. Chem. 396, 759–765 (2015).
Realini, N. et al. Acid ceramidase in melanoma: expression, localization, and effects of pharmacological inhibition. J. Biol. Chem. 291, 2422–2434 (2016).
Bizzozero, L. et al. Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ. 21, 507–520 (2014).
Sanger, N. et al. Acid ceramidase is associated with an improved prognosis in both DCIS and invasive breast cancer. Mol. Oncol. 9, 58–67 (2015).
Carpinteiro, A. et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol. Med. 7, 714–734 (2015).
Truman, J. P. et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS ONE 5, e12310 (2010).
Daemen, A. et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc. Natl Acad. Sci. USA 112, E4410–E4417 (2015).
Dubois, N. et al. Plasma ceramide, a real-time predictive marker of pulmonary and hepatic metastases response to stereotactic body radiation therapy combined with irinotecan. Radiother. Oncol. 119, 229–235 (2016).
Abdul Aziz, N. A. et al. 19-Gene expression signature as a predictor of survival in colorectal cancer. BMC Med. Genom. 9, 58 (2016). This study identifies CERS6 as a key gene component of a 19-gene signature for prediction of survival in colon cancer.
Kasumov, T. et al. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS ONE 10, e0126910 (2015).
Boini, K. M., Zhang, C., Xia, M., Poklis, J. L. & Li, P. L. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 334, 839–846 (2010).
Choi, S. & Snider, A. J. Sphingolipids in high fat diet and obesity-related diseases. Mediators Inflamm. 2015, 520618 (2015).
Hodson, A. E., Tippetts, T. S. & Bikman, B. T. Insulin treatment increases myocardial ceramide accumulation and disrupts cardiometabolic function. Cardiovasc. Diabetol. 14, 153 (2015).
Kurek, K. et al. Inhibition of ceramide de novo synthesis with myriocin affects lipid metabolism in the liver of rats with streptozotocin-induced type 1 diabetes. BioMed Res. Int. 2014, 980815 (2014).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Xia, J. Y. et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278 (2015).
Chavez, J. A. et al. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem. 289, 723–734 (2014).
Li, Z. et al. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol. Cell. Biol. 31, 4205–4218 (2011).
Yano, M. et al. Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS ONE 8, e61380 (2013).
Taguchi, Y. et al. Sphingosine-1-phosphate phosphatase 2 regulates pancreatic islet β-cell endoplasmic reticulum stress and proliferation. J. Biol. Chem. 291, 12029–12038 (2016).
Chen, J. et al. Deletion of sphingosine kinase 1 ameliorates hepatic steatosis in diet-induced obese mice: role of PPARγ. Biochim. Biophys. Acta 1861, 138–147 (2016).
Park, K. et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc. Natl Acad. Sci. USA 113, E1334–E1342 (2016).
Wong, M. L. et al. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: A possible link between inflammatory cytokines and atherogenesis. Proc. Natl Acad. Sci. USA 97, 8681–8686 (2000).
Fan, J., Wu, B. X. & Crosson, C. E. Suppression of acid sphingomyelinase protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 57, 4476–4484 (2016).
Reforgiato, M. R. et al. Inhibition of ceramide de novo synthesis as a postischemic strategy to reduce myocardial reperfusion injury. Basic Res. Cardiol. 111, 12 (2016).
Hammad, S. M. et al. Increased plasma levels of select deoxy-ceramide and ceramide species are associated with increased odds of diabetic neuropathy in type 1 diabetes: a pilot study. Neuromolecular Med. 19, 46–56 (2017).
Havulinna, A. S. et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 36, 2424–2430 (2016).
Cheng, J. M. et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 243, 560–566 (2015).
Sigruener, A. et al. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE 9, e85724 (2014).
Saleem, M. et al. Ceramides predict verbal memory performance in coronary artery disease patients undertaking exercise: a prospective cohort pilot study. BMC Geriatr. 13, 135 (2013).
Soltau, I. et al. Serum-sphingosine-1-phosphate concentrations are inversely associated with atherosclerotic diseases in humans. PLoS ONE 11, e0168302 (2016).
Othman, A. et al. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 3, e000073 (2015).
Hama, H. Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta 1801, 405–414 (2010).
Edvardson, S. et al. Deficiency of the alkaline ceramidase ACER3 manifests in early childhood by progressive leukodystrophy. J. Med. Genet. 53, 389–396 (2016).
Zhao, L. et al. Elevation of 20-carbon long chain bases due to a mutation in serine palmitoyltransferase small subunit b results in neurodegeneration. Proc. Natl Acad. Sci. USA 112, 12962–12967 (2015).
Vanni, N. et al. Impairment of ceramide synthesis causes a novel progressive myoclonus epilepsy. Ann. Neurol. 76, 206–212 (2014).
Mosbech, M. B. et al. Reduced ceramide synthase 2 activity causes progressive myoclonic epilepsy. Ann. Clin. Transl Neurol. 1, 88–98 (2014).
Boustany, R. M. Ceramide center stage in progressive myoclonus epilepsies. Ann. Neurol. 76, 162–164 (2014).
Spassieva, S. D. et al. Ectopic expression of ceramide synthase 2 in neurons suppresses neurodegeneration induced by ceramide synthase 1 deficiency. Proc. Natl Acad. Sci. USA 113, 5928–5933 (2016). This study, by using genetic interactions between Cers1 and Cers2 , demonstrates that sphingosine is likely the key lipid species responsible for mediating neurodegeneration in the Cers1 -knockout mouse.
Dinkins, M. B. et al. Neutral sphingomyelinase-2 deficiency ameliorates Alzheimer's disease pathology and improves cognition in the 5XFAD mouse. J. Neurosci. 36, 8653–8667 (2016).
Novgorodov, S. A. et al. Essential roles of neutral ceramidase and sphingosine in mitochondrial dysfunction due to traumatic brain injury. J. Biol. Chem. 289, 13142–13154 (2014).
Jennemann, R. et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 21, 586–608 (2012).
Behne, M. et al. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J. Invest. Dermatol. 114, 185–192 (2000).
Jennemann, R. et al. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J. Biol. Chem. 282, 3083–3094 (2007).
Westerberg, R. et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J. Biol. Chem. 279, 5621–5629 (2004).
Cameron, D. J. et al. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int. J. Biol. Sci. 3, 111–119 (2007).
Peters, F. et al. Ceramide synthase 4 regulates stem cell homeostasis and hair follicle cycling. J. Invest. Dermatol. 135, 1501–1509 (2015).
Liakath-Ali, K. et al. Alkaline ceramidase 1 is essential for mammalian skin homeostasis and regulating whole-body energy expenditure. J. Pathol. 239, 374–383 (2016).
Stoffel, W., Jenke, B., Block, B., Zumbansen, M. & Koebke, J. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc. Natl Acad. Sci. USA 102, 4554–4559 (2005).
Li, J. et al. Smpd3 expression in both chondrocytes and osteoblasts is required for normal endochondral bone development. Mol. Cell. Biol. 36, 2282–2299 (2016).
Kakoi, H. et al. Bone morphogenic protein (BMP) signaling up-regulates neutral sphingomyelinase 2 to suppress chondrocyte maturation via the Akt protein signaling pathway as a negative feedback mechanism. J. Biol. Chem. 289, 8135–8150 (2014).
Somenzi, G. et al. Disruption of retinoic acid receptor alpha reveals the growth promoter face of retinoic acid. PLoS ONE 2, e836 (2007).
Clarke, C. J. et al. ATRA transcriptionally induces nSMase2 through CBP/p300-mediated histone acetylation. J. Lipid Res. 57, 868–881 (2016).
Cowart, L. A. & Hannun, Y. A. Selective substrate supply in the regulation of yeast de novo sphingolipid synthesis. J. Biol. Chem. 282, 12330–12340 (2007).
Sun, Y. et al. Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Mol. Biol. Cell 23, 2388–2398 (2012).
Muir, A., Ramachandran, S., Roelants, F. M., Timmons, G. & Thorner, J. TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. eLife 3, e03779 (2014).
Novgorodov, S. A. et al. SIRT3 deacetylates ceramide synthases: implications for mitochondrial dysfunction and brain injury. J. Biol. Chem. 291, 1957–1973 (2016).
Sassa, T., Hirayama, T. & Kihara, A. Enzyme activities of the ceramide synthases CERS2-6 are regulated by phosphorylation in the C-terminal region. J. Biol. Chem. 291, 7477–7487 (2016).
Jensen, S. A. et al. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl Acad. Sci. USA 111, 5682–5687 (2014).
McNaughton, M., Pitman, M., Pitson, S. M., Pyne, N. J. & Pyne, S. Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKi or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget 7, 16663–16675 (2016).
Filosto, S., Ashfaq, M., Chung, S., Fry, W. & Goldkorn, T. Neutral sphingomyelinase 2 activity and protein stability are modulated by phosphorylation of five conserved serines. J. Biol. Chem. 287, 514–522 (2012).
Shamseddine, A. A., Airola, M. V. & Hannun, Y. A. Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Adv. Biol. Reg. 57, 24–41 (2015).
Rhein, C. et al. Functional implications of novel human acid sphingomyelinase splice variants. PLoS ONE 7, e35467 (2012).
Sasaki, H. et al. Regulation of alkaline ceramidase activity by the c-Src-mediated pathway. Arch. Biochem. Biophys. 550–551, 12–19 (2014).
Tanaka, K. et al. Role of down-regulated neutral ceramidase during all-trans retinoic acid-induced neuronal differentiation in SH-SY5Y neuroblastoma cells. J. Biochem. 151, 611–620 (2012).
Wu, B. X., Zeidan, Y. H. & Hannun, Y. A. Downregulation of neutral ceramidase by gemcitabine: Implications for cell cycle regulation. Biochim. Biophys. Acta 1791, 730–739 (2009).
Rahmaniyan, M. et al. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J. Biol. Chem. 286, 24754–24764 (2011). This study identifies dihydroceramide desaturase, the enzyme responsible for introducing the 4–5 double bond into ceramide, as a direct target for the action of the chemotherapeutic agent fenretinide (4-HPR).
Schnute, M. E. et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 444, 79–88 (2012).
Rex, K. et al. Sphingosine kinase activity is not required for tumor cell viability. PLoS ONE 8, e68328 (2013).
Santos, W. L. & Lynch, K. R. Drugging sphingosine kinases. ACS Chem. Biol. 10, 225–233 (2015).
Realini, N. et al. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Sci. Rep. 3, 1035 (2013).
Sandborn, W. J. et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N. Engl. J. Med. 374, 1754–1762 (2016).
Zhang, L. et al. Anti-S1P antibody as a novel therapeutic strategy for VEGFR TKI-resistant renal cancer. Clin. Cancer Res. 21, 1925–1934 (2015).
Rollin-Pinheiro, R., Singh, A., Barreto-Bergter, E. & Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 8, 1469–1484 (2016).
Kumagai, K., Kawano-Kawada, M. & Hanada, K. Phosphoregulation of the ceramide transport protein CERT at serine 315 in the interaction with VAMP-associated protein (VAP) for inter-organelle trafficking of ceramide in mammalian cells. J. Biol. Chem. 289, 10748–10760 (2014).
D'Angelo, G. et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449, 62–67 (2007). This study identifies that the transfer protein FAPP2 is involved in the selective binding and transport of neutral glycolipids among Golgi cisternae.
Acknowledgements
The authors thank the members of their laboratories and M. Airola, C. Luberto, C. Clarke, D. Canals, C. Senkal, C. Rhein and F. Velazquez for helpful discussions. The authors are very grateful for the contribution of M. Hernandez for assembling table 1 and supplementary information tables S2 and S3. Due to space limitations, the authors have striven to reference the more recent studies pertinent to the presentation while directing the readers to more in-depth targeted reviews. The authors apologize for the multitude of sphingolipid investigators whose works they could not cite in this Review.
Author information
Authors and Affiliations
Contributions
Both authors conducted extensive research of primary data and reviews for the article and collaborated in defining the scope and content of the Review, in writing the Review and in preparation of the figures and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (box)
Studying sphingolipids: limitations and technical advancements. (PDF 59 kb)
Supplementary information S2 (table)
Sphingolipid species and key cellular functions (PDF 402 kb)
Supplementary information S3 (table)
Sphingolipid metabolizing enzymes (PDF 315 kb)
Glossary
- Prostaglandins
-
Bioactive, acidic lipids with various hormone-like activities, including modulation of inflammation, regulation of blood flow and blood pressure and reproduction.
- Gangliosides
-
A major subtype of sphingolipids composed of ceramide and an oligosaccharide that contains at least one sialic acid residue.
- ω-Acylation
-
Acylation of fatty acids at the ω-position (last position) in the acyl chain.
- Freeze–fracture studies
-
A form of electron microscopy involving freezing in order to preserve lipid membrane structures.
- G protein-coupled receptors
-
(GPCRs). Heptahelical membrane receptors that bind and regulate G proteins.
- Globosides
-
A subtype of sphingolipids with a ceramide associated with at least two sugars, but no sialic acid.
- Diauxic shift
-
The shift in growth from rapid fermentative to aerobic glycolysis.
- Microglia
-
A type of neural cell arising from macrophages or their precursors that serves supportive and protective functions in the central nervous system.
- ER stress
-
Endoplasmic reticulum (ER) dysfunction due to stress stimuli that result in increased accumulation of misfolded proteins in the ER.
- Necroptosis
-
A regulated form of necrotic cell death associated with immune and inflammatory responses.
- Multiple sclerosis
-
A degenerative disease of the nervous system associated with a loss of myelination (covering) of axonal sheaths.
- T cell egress
-
Lymphocyte migration from the thymus and lymph nodes into the bloodstream.
- von Hippel–Lindau (VHL) gene
-
A gene whose mutations can result in von Hippel–Lindau disease. It encodes a protein that participates in the regulation of the levels of hypoxia-inducible factor (HIF) through degradation.
- Luminal A type breast cancer
-
A subtype of breast cancer in which the cells appear to resemble most cells of the luminal lining of the breast ducts.
- Insulin resistance
-
A state in which cells, tissues or organisms fail to respond normally to insulin.
- Glucose intolerance
-
Also known as impaired glucose tolerance. A pre-diabetic state involving hyperglycaemia and usually poor responsiveness to insulin.
- Hepatic steatosis
-
Condition associated with non-alcoholic fatty liver disease with increased accumulation of fat in liver cells, usually in the form of triglycerides.
- Ischaemic injury
-
Tissue and cell injury that results from a decrease in or interruption of the blood supply.
- Reperfusion injury
-
Injury or damage to tissues resulting from the reoxygenation of previously ischaemic tissues.
- Diabetic neuropathy
-
Dysfunction of the peripheral and autonomic nervous system that arises from long-standing diabetes.
- Coronary angiography
-
An invasive procedure using dyes in the bloodstream to visualize the coronary circulation using radiography.
- Osteogenesis imperfecta
-
A group of genetic disorders characterized by brittle bones.
Rights and permissions
About this article
Cite this article
Hannun, Y., Obeid, L. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19, 175–191 (2018). https://doi.org/10.1038/nrm.2017.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2017.107
This article is cited by
-
Lipid mediated plant immunity in susceptible and tolerant soybean cultivars in response to Phytophthora sojae colonization and infection
BMC Plant Biology (2024)
-
The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: a promising therapeutic target
Cancer Cell International (2024)
-
SPHK1 potentiates colorectal cancer progression and metastasis via regulating autophagy mediated by TRAF6-induced ULK1 ubiquitination
Cancer Gene Therapy (2024)
-
Cryo-EM structure of human sphingomyelin synthase and its mechanistic implications for sphingomyelin synthesis
Nature Structural & Molecular Biology (2024)
-
A modular chemoenzymatic cascade strategy for the structure-customized assembly of ganglioside analogs
Communications Chemistry (2024)