Key Points
-
The heparan sulphate proteoglycan agrin, which is encoded by a single gene, is known to be a key regulator of the formation of postsynaptic structures at the neuromuscular junction (NMJ).
-
Alternative messenger RNA splicing gives rise to several agrin isoforms that differ at their amino and/or their carboxyl termini. Whereas splicing at the amino terminus might affect agrin's localization in various tissues, carboxy-terminal splicing regulates agrin's ability to induce postsynapses.
-
Agrin's ability to induce postsynaptic structures at the NMJ requires the muscle-specific receptor tyrosine kinase MuSK. Agrin- and MuSK-deficient mice have similar phenotypes and die at birth. Biochemical studies indicate that MuSK does not bind agrin directly, but rather is a signalling component of a larger protein complex, the components of which have not yet been identified.
-
Agrin is also expressed in the brain but its exact role in this organ is not known.
-
The recently described role of agrin in T-cell activation indicates that agrin's function might be regulated by glycosylation, and that agrin might be involved in the aggregation of lipid rafts at the immunological synapse.
-
At the NMJ, agrin also activates the Rho-family of small GTPases, indicating that the cytoskeleton has an active role in the formation of postsynapses. The adaptor protein Dishevelled seems to regulate the specificity and efficiency of this activation.
-
The agrin splice variant that is inactive in inducing postsynapses at the NMJ has recently been shown to globally affect the cytoskeleton of skeletal muscle through costamere organization. Moreover, a miniature version of this isoform ameliorates the dystrophic phenotype in mice that lack the laminin α2 chain, which is an animal model for merosin-deficient congenital muscle dystrophy.
-
Further studies that are focused on agrin glycosylation, its role in the aggregation of lipid rafts and cytoskeletal organization might unravel some of the missing links in our knowledge of the formation of synapses in the nervous and immune systems.
Abstract
The heparan sulphate proteoglycan agrin is expressed as several isoforms in various tissues. Agrin is best known as a crucial organizer of postsynaptic differentiation at the neuromuscular junction, but it has recently also been implicated in the formation of the immunological synapse, the organization of the cytoskeleton and the amelioration of function in diseased muscle. So the activities of agrin might be of broader significance than previously anticipated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sherrington, C. S. in A Text-Book of Physiology 7th edn (ed. Foster, M.) Vol. 3 (London, 1897).
Paul, W. E. & Seder, R. A. Lymphocyte responses and cytokines. Cell 76, 241–251 (1994).
Benson, D. L., Colman, D. R. & Huntley, G. W. Molecules, maps and synapse specificity. Nature Rev. Neurosci. 2, 899–909 (2001).
Sanes, J. R. & Lichtman, J. W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Rev. Neurosci. 2, 791–805 (2001).
Dustin, M. L. & Chan, A. C. Signaling takes shape in the immune system. Cell 103, 283–294 (2000).
Ruegg, M. A. & Bixby, J. L. Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 21, 22–27 (1998).
Khan, A. A., Bose, C., Yam, L. S., Soloski, M. J. & Rupp, F. Physiological regulation of the immunological synapse by agrin. Science 292, 1681–1686 (2001). The first demonstration that agrin is expressed in T cells and that it can function as a co-stimulatory signal in T-cell activation. Agrin isolated from activated T cells also induces the aggregation of AChRs and lipid rafts on cultured muscle cells.
Bezakova, G. & Lømo, T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers. J. Cell Biol. 153, 1453–1463 (2001). This article shows that recombinant agrin-B/z− injected into innervated or denervated muscle regulates the organization of cytoskeletal proteins in skeletal muscle fibres. This is the first evidence for a function of agrin–B/z− in regulating the cytoskeleton.
Moll, J. et al. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 413, 302–307 (2001). By generating transgenic mice overexpressing a miniaturized form of agrin-B/z− in skeletal muscle, these authors show that mini-agrin can substitute for the loss of laminin-2. This study is the first evidence that related proteins can compensate for each other.
Sanes, J. R., Marshall, L. M. & McMahan, U. J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J. Cell Biol. 78, 176–198 (1978).
Burden, S. J., Sargent, P. B. & McMahan, U. J. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J. Cell Biol. 82, 412–425 (1979).
Nitkin, R. M. et al. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol. 105, 2471–2478 (1987).
McMahan, U. J. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 55, 407–418 (1990). This review formulates, for the first time, the idea that agrin derived from motor neurons organizes the entire postsynaptic apparatus at the NMJ.
Gautam, M. et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535 (1996). By generating an agrin-deficient mouse, these authors provide compelling evidence that agrin is necessary for the formation of the NMJ.
Jones, G. et al. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc. Natl Acad. Sci. USA 94, 2654–2659 (1997). This reference provides the first evidence that agrin-B/z+ regulates expression of AChR ε-subunit.
Bezakova, G., Helm, J. P., Francolini, M. & Lømo, T. Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo. J. Cell Biol. 153, 1441–1452 (2001). Injection of recombinant protein, or expression plasmids encoding agrin-B/z+ (reference 15), into the soleus muscle of rodents initiates formation of postsynaptic structures that are indistinguishable from those found at the neuromuscular junction.
Tsen, G., Halfter, W., Kroger, S. & Cole, G. J. Agrin is a heparan sulfate proteoglycan. J. Biol. Chem. 270, 3392–3399 (1995).
Rupp, F., Payan, D. G., Magill-Solc, C., Cowan, D. M. & Scheller, R. H. Structure and expression of a rat agrin. Neuron 6, 811–823 (1991).
Tsim, K. W. K., Ruegg, M. A., Escher, G., Kröger, S. & McMahan, U. J. cDNA that encodes active agrin. Neuron 8, 677–689 (1992).
Denzer, A. J., Gesemann, M., Schumacher, B. & Ruegg, M. A. An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J. Cell Biol. 131, 1547–1560 (1995).
Burgess, R. W., Skarnes, W. C. & Sanes, J. R. Agrin isoforms with distinct amino termini. Differential expression, localization, and function. J. Cell Biol. 151, 41–52 (2000).
Neumann, F. R. et al. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Mol. Cell. Neurosci. 17, 208–225 (2001).
Denzer, A. J., Brandenberger, R., Gesemann, M., Chiquet, M. & Ruegg, M. A. Agrin binds to the nerve–muscle basal lamina via laminin. J. Cell Biol. 137, 671–683 (1997).
Gesemann, M. et al. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron 16, 755–767 (1996).
Hopf, C. & Hoch, W. Agrin binding to α-dystroglycan — domains of agrin necessary to induce acetylcholine receptor clustering are overlapping but not identical to the α-dystroglycan-binding region. J. Biol. Chem. 271, 5231–5236 (1996).
Burgess, R. W., Nguyen, Q. T., Son, Y. J., Lichtman, J. W. & Sanes, J. R. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23, 33–44 (1999). By generating knockouts of the exons coding for the inserts in the B/z site of agrin, these authors show that the presence of these amino-acid inserts is required for the formation of postsynaptic structures at the NMJ.
Yang, J. F., Cao, G., Koirala, S., Reddy, L. V. & Ko, C. P. Schwann cells express active agrin and enhance aggregation of acetylcholine receptors on muscle fibers. J. Neurosci. 21, 9572–9584 (2001).
Gesemann, M., Denzer, A. J. & Ruegg, M. A. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J. Cell Biol. 128, 625–636 (1995).
Cornish, T., Chi, J., Johnson, S., Lu, Y. & Campanelli, J. T. Globular domains of agrin are functional units that collaborate to induce acetylcholine receptor clustering. J. Cell Sci. 112, 1213–1223 (1999).
Sanes, J. R. & Lichtman, J. W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Bowe, M. A., Deyst, K. A., Leszyk, J. D. & Fallon, J. R. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron 12, 1173–1180 (1994).
Gee, S. H., Montanaro, F., Lindenbaum, M. H. & Carbonetto, S. Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell 77, 675–686 (1994).
Campanelli, J. T., Roberds, S. L., Campbell, K. P. & Scheller, R. H. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell 77, 663–674 (1994).
Sugiyama, J., Bowen, D. C. & Hall, Z. W. Dystroglycan binds nerve and muscle agrin. Neuron 13, 103–115 (1994).
Cote, P. D., Moukhles, H., Lindenbaum, M. & Carbonetto, S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nature Genet. 23, 338–342 (1999).
Grady, R. M. et al. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron 25, 279–293 (2000).
Jacobson, C., Cote, P. D., Rossi, S. G., Rotundo, R. L. & Carbonetto, S. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J. Cell Biol. 152, 435–450 (2001).
DeChiara, T. M. et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85, 501–512 (1996). By generating a MuSK-deficient mouse, these authors provide compelling evidence that MuSK is necessary for the formation of the NMJ. The high similarity with the phenotype of agrin-deficient mice indicates that MuSK is part of the receptor complex activated by agrin-B/z+.
Herbst, R., Avetisova, E. & Burden, S. J. Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development 129, 5449–5460 (2002).
Glass, D. J. et al. Agrin acts via a MuSK receptor complex. Cell 85, 513–523 (1996).
Smith, M. A. & Hilgenberg, L. G. Agrin in the CNS: a protein in search of a function? Neuroreport 13, 1485–1495 (2002).
Gingras, J., Rassadi, S., Cooper, E. & Ferns, M. Agrin plays an organizing role in the formation of sympathetic synapses. J. Cell Biol. 158, 1109–1118 (2002).
Gingras, J. & Ferns, M. Expression and localization of agrin during sympathetic synapse formation in vitro. J. Neurobiol. 48, 228–242 (2001).
Ip, F. C. et al. Cloning and characterization of muscle-specific kinase in chicken. Mol. Cell. Neurosci. 16, 661–673 (2000).
Ferreira, A. Abnormal synapse formation in agrin-depleted hippocampal neurons. J. Cell Sci. 112, 4729–4738 (1999).
Bose, C. M. et al. Agrin controls synaptic differentiation in hippocampal neurons. J. Neurosci. 20, 9086–9095 (2000).
Ji, R. R. et al. Specific agrin isoforms induce cAMP response element binding protein phosphorylation in hippocampal neurons. J. Neurosci. 18, 9695–9702 (1998).
Hilgenberg, L. G., Hoover, C. L. & Smith, M. A. Evidence of an agrin receptor in cortical neurons. J. Neurosci. 19, 7384–7393 (1999).
Campagna, J. A., Ruegg, M. A. & Bixby, J. L. Agrin is a differentiation-inducing 'stop signal' for motoneurons in vitro. Neuron 15, 1365–1374 (1995). This work provides the first evidence for a role of agrin in the differentiation of a presynaptic nerve terminal. This activity is in agreement with the observation, in reference 14, that motor neurons do not form presynaptic nerve terminals in agrin-deficient mice.
Cotman, S. L., Halfter, W. & Cole, G. J. Agrin binds to β-amyloid (Aβ), accelerates Aβ fibril formation, and is localized to Aβ deposits in Alzheimer's disease brain. Mol. Cell. Neurosci. 15, 183–198 (2000).
Donahue, J. E. et al. Agrin in Alzheimer's disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc. Natl Acad. Sci. USA 96, 6468–6472 (1999).
Serpinskaya, A. S., Feng, G., Sanes, J. R. & Craig, A. M. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev. Biol. 205, 65–78 (1999).
Li, Z., Hilgenberg, L. G., O'Dowd, D. K. & Smith, M. A. Formation of functional synaptic connections between cultured cortical neurons from agrin-deficient mice. J. Neurobiol. 39, 547–557 (1999).
Ruegg, M. A. et al. The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron 8, 691–699 (1992).
Hoch, W., Campanelli, J. T., Harrison, S. & Scheller, R. H. Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO J. 13, 2814–2821 (1994).
Martin, P. T. & Sanes, J. R. Role for a synapse-specific carbohydrate in agrin-induced clustering of acetylcholine receptors. Neuron 14, 743–754 (1995).
Grow, W. A., Ferns, M. & Gordon, H. Agrin-independent activation of the agrin signal transduction pathway. J. Neurobiol. 40, 356–365 (1999).
Grow, W. A. & Gordon, H. Sialic acid inhibits agrin signaling in C2 myotubes. Cell Tissue Res. 299, 273–279 (2000).
Xia, B. & Martin, P. T. Modulation of agrin binding and activity by the CT and related carbohydrate antigens. Mol. Cell. Neurosci. 19, 539–551 (2002).
Xia, B. et al. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev. Biol. 242, 58–73 (2002).
Harris, A. J. Embryonic growth and innervation of rat skeletal muscles. III. Neural regulation of junctional and extra-junctional acetylcholine receptor clusters. Phil. Trans. R. Soc. Lond. B 293, 287–314 (1981).
Yang, X., Li, W., Prescott, E. D., Burden, S. J. & Wang, J. C. DNA topoisomerase IIβ and neural development. Science 287, 131–134 (2000).
Lin, W. et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057–1064 (2001).
Yang, X. et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 399–410 (2001).
Schaeffer, L., de Kerchove d'Exaerde, A. & Changeux, J. P. Targeting transcription to the neuromuscular synapse. Neuron 31, 15–22 (2001).
Fischbach, G. D. & Cohen, S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev. Biol. 31, 147–162 (1973).
Jasmin, B. J. et al. Multiple regulatory events controlling the expression and localization of utrophin in skeletal muscle fibers: insights into a therapeutic strategy for Duchenne muscular dystrophy. J. Physiol. (Paris) 96, 31–42 (2002).
Dai, Z., Luo, X., Xie, H. & Peng, H. B. The actin-driven movement and formation of acetylcholine receptor clusters. J. Cell Biol. 150, 1321–1334 (2000).
Weston, C., Yee, B., Hod, E. & Prives, J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J. Cell Biol. 150, 205–212 (2000).
Uhm, C. S., Neuhuber, B., Lowe, B., Crocker, V. & Daniels, M. P. Synapse-forming axons and recombinant agrin induce microprocess formation on myotubes. J. Neurosci. 21, 9678–9689 (2001).
Wallace, B. G., Qu, Z. & Huganir, R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron 6, 869–878 (1991).
Meyer, G. & Wallace, B. G. Recruitment of a nicotinic acetylcholine receptor mutant lacking cytoplasmic tyrosine residues in its β subunit into agrin-induced aggregates. Mol. Cell. Neurosci. 11, 324–333 (1998).
Ferns, M., Deiner, M. & Hall, Z. Agrin-induced acetylcholine receptor clustering in mammalian muscle requires tyrosine phosphorylation. J. Cell Biol. 132, 937–944 (1996).
Borges, L. S. & Ferns, M. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol. 153, 1–12 (2001).
Mohamed, A. S. & Swope, S. L. Phosphorylation and cytoskeletal anchoring of the acetylcholine receptor by Src class protein-tyrosine kinases. Activation by rapsyn. J. Biol. Chem. 274, 20529–20539 (1999).
Luo, Z. et al. Regulation of AChR clustering by dishevelled interacting with MuSK and PAK1. Neuron 35, 489–505 (2002). This article provides direct evidence for MuSK involvement in regulating p21-activated kinase (PAK). The activation is triggered by agrin-B/z+ and requires the adaptor protein Dishevelled, which binds to MuSK.
Apel, E. D., Roberds, S. L., Campbell, K. P. & Merlie, J. P. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron 15, 115–126 (1995).
Moon, R. T., Bowerman, B., Boutros, M. & Perrimon, N. The promise and perils of Wnt signaling through β-catenin. Science 296, 1644–1646 (2002).
Peng, H. B., Xie, H. & Dai, Z. Association of cortactin with developing neuromuscular specializations. J. Neurocytol. 26, 637–650 (1997).
Boeckers, T. M. et al. Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J. Neurosci. 19, 6506–6518 (1999).
Taniuchi, I. et al. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen-receptor-coupled tyrosine kinases. EMBO J. 14, 3664–3678 (1995).
Weed, S. A. & Parsons, J. T. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20, 6418–6434 (2001).
Weed, S. A., Du, Y. & Parsons, J. T. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111, 2433–2443 (1998).
Gesemann, M., Brancaccio, A., Schumacher, B. & Ruegg, M. A. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J. Biol. Chem. 273, 600–605 (1998).
Burgess, R. W., Dickman, D. K., Nunez, L., Glass, D. J. & Sanes, J. R. Mapping sites responsible for interactions of agrin with neurons. J. Neurochem. 83, 271–284 (2002).
Berthier, C. & Blaineau, S. Supramolecular organization of the subsarcolemmal cytoskeleton of adult skeletal muscle fibers. A review. Biol. Cell 89, 413–434 (1997).
Colognato, H. & Yurchenco, P. D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213–234 (2000).
Durbeej, M. & Campbell, K. P. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 12, 349–361 (2002).
Williams, M. W. & Bloch, R. J. Extensive but coordinated reorganization of the membrane skeleton in myofibers of dystrophic (mdx) mice. J. Cell Biol. 144, 1259–1270 (1999).
Geiger, B. & Bershadsky, A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 13, 584–592 (2001).
Geiger, B. & Bershadsky, A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell 110, 139–142 (2002).
Sanders, L. C., Matsumura, F., Bokoch, G. M. & de Lanerolle, P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283, 2083–2085 (1999).
van Leeuwen, F. N., van Delft, S., Kain, H. E., van der Kammen, R. A. & Collard, J. G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nature Cell Biol. 1, 242–248 (1999).
Volberg, T., Romer, L., Zamir, E. & Geiger, B. pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J. Cell Sci. 114, 2279–2289 (2001).
Suen, P. W. et al. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J. Cell Sci. 112, 4067–4078 (1999).
Smith, C. L., Mittaud, P., Prescott, E. D., Fuhrer, C. & Burden, S. J. Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. J. Neurosci. 21, 3151–3160 (2001).
Briguet, A. & Ruegg, M. A. The ets transcription factor GABP is required for postsynaptic differentiation in vivo. J. Neurosci. 20, 5989–5996 (2000).
Megeath, L. J. & Fallon, J. R. Intracellular calcium regulates agrin-induced acetylcholine receptor clustering. J. Neurosci. 18, 672–678 (1998).
Dustin, M. L. & Cooper, J. A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nature Immunol. 1, 23–29 (2000).
Geberhiwot, T. et al. Laminin-8 (α4β1γ1) is synthesized by lymphoid cells, promotes lymphocyte migration and costimulates T cell proliferation. J. Cell Sci. 114, 423–433 (2001).
Foger, N., Marhaba, R. & Zoller, M. Involvement of CD44 in cytoskeleton rearrangement and raft reorganization in T cells. J. Cell Sci. 114, 1169–1178 (2001).
Ponta, H., Sherman, L. & Herrlich, P. A. CD44: from adhesion molecules to signalling regulators. Nature Rev. Mol. Cell Biol. 4, 33–45 (2003).
del Pozo, M. A. et al. The two poles of the lymphocyte: specialized cell compartments for migration and recruitment. Cell. Adhes. Commun. 6, 125–133 (1998).
Katoh, S., Zheng, Z., Oritani, K., Shimozato, T. & Kincade, P. W. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J. Exp. Med. 182, 419–429 (1995).
Gautam, M. et al. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377, 232–236 (1995).
Marchand, S., Bignami, F., Stetzkowski-Marden, F. & Cartaud, J. The myristoylated protein rapsyn is cotargeted with the nicotinic acetylcholine receptor to the postsynaptic membrane via the exocytic pathway. J. Neurosci. 20, 521–528 (2000).
Marchand, S., Devillers-Thiery, A., Pons, S., Changeux, J. P. & Cartaud, J. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J. Neurosci. 22, 8891–8901 (2002).
Apel, E. D., Glass, D. J., Moscoso, L. M., Yancopoulos, G. D. & Sanes, J. R. Rapsyn is required for MuSK signalling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18, 623–635 (1997).
Mittaud, P., Marangi, P. A., Erb-Vogtli, S. & Fuhrer, C. Agrin-induced activation of acetylcholine receptor-bound Src family kinases requires Rapsyn and correlates with acetylcholine receptor clustering. J. Biol. Chem. 276, 14505–14513 (2001).
Cartaud, A., Coutant, S., Petrucci, T. C. & Cartaud, J. Evidence for in situ and in vitro association between β-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J. Biol. Chem. 273, 11321–11326 (1998).
Colicos, M. A., Collins, B. E., Sailor, M. J. & Goda, Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell 107, 605–616 (2001).
Cole, G. J. & Halfter, W. Agrin: an extracellular matrix heparan sulfate proteoglycan involved in cell interactions and synaptogenesis. Perspect. Dev. Neurobiol. 3, 359–371 (1996).
Rupp, F. et al. Structure and chromosomal localization of the mammalian agrin gene. J. Neurosci. 12, 3535–3544 (1992).
Ferns, M. J., Campanelli, J. T., Hoch, W., Scheller, R. H. & Hall, Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron 11, 491–502 (1993).
Winder, S. J. The complexities of dystroglycan. Trends Biochem. Sci. 26, 118–124 (2001).
Williamson, R. A. et al. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum. Mol. Genet. 6, 831–841 (1997).
Michele, D. E. et al. Post-translational disruption of dystroglycan ligand interactions in congenital muscular dystrophies. Nature 418, 417–421 (2002).
Jennings, C. G., Dyer, S. M. & Burden, S. J. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc. Natl Acad. Sci. USA 90, 2895–2899 (1993).
Valenzuela, D. M. et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 15, 573–584 (1995).
Ganju, P., Walls, E., Brennan, J. & Reith, A. D. Cloning and developmental expression of Nsk2, a novel receptor tyrosine kinase implicated in skeletal myogenesis. Oncogene 11, 281–290 (1995).
Herbst, R. & Burden, S. J. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 19, 67–77 (2000).
Hoch, W. et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nature Med. 7, 365–368 (2001).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nature Rev. Mol. Cell Biol. 1, 31–39 (2000).
Devor, A., Fritschy, J. M. & Yarom, Y. Spatial distribution and subunit composition of GABAA receptors in the inferior olivary nucleus. J. Neurophysiol. 85, 1686–1696 (2001).
Turner, C. E. et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145, 851–863 (1999).
Ma, E., Morgan, R. & Godfrey, E. W. Distribution of agrin mRNAs in the chick embryo nervous system. J. Neurosci. 14, 2943–2952 (1994).
Stone, D. M. & Nikolics, K. Tissue- and age-specific expression patterns of alternatively spliced agrin mRNA transcripts in embryonic rat suggest novel developmental roles. J. Neurosci. 15, 6767–6778 (1995).
Hoch, W., Ferns, M., Campanelli, J. T., Hall, Z. W. & Scheller, R. H. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron 11, 479–490 (1993).
Ma, E., Morgan, R. & Godfrey, E. W. Agrin mRNA variants are differentially regulated in developing chick embryo spinal cord and sensory ganglia. J. Neurobiol. 26, 585–597 (1995).
O'Connor, L. T., Lauterborn, J. C., Gall, C. M. & Smith, M. A. Localization and alternative splicing of agrin mRNA in adult rat brain: transcripts encoding isoforms that aggregate acetylcholine receptors are not restricted to cholinergic regions. J. Neurosci. 14, 1141–1152 (1994).
Smith, M. A. & O'Dowd, D. K. Cell-specific regulation of agrin RNA splicing in the chick ciliary ganglion. Neuron 12, 795–804 (1994).
Kroger, S., Horton, S. E. & Honig, L. S. The developing avian retina expresses agrin isoforms during synaptogenesis. J. Neurobiol. 29, 165–182 (1996).
Kroger, S. Differential distribution of agrin isoforms in the developing and adult avian retina. Mol. Cell. Neurosci. 10, 149–161 (1997).
Acknowledgements
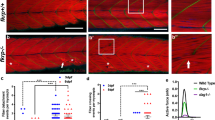
We would like to thank J.L. Bixby, T. Meier & A. Rolink for their critical reading of this manuscript. We are grateful to A. Devor and J.M. Fritschy for providing figure 1a, and to F. Rupp for providing figure 1c. We would also like to apologize to all of our colleagues whose contributions to the work summarized in this review could not be cited because of space constraints. The work in our laboratory is supported by the Kanton Basel-Stadt, the Swiss National Science Foundation, the Swiss Foundation for Research on Muscle Diseases and the Muscular Dystrophy Association USA.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- ANTIGEN-PRESENTING CELL
-
A cell that is specialized in the generation of epitopes that are presented through major histocompatibility complex (MHC) I or II to T lymphocytes.
- NEUROMUSCULAR JUNCTION
-
(NMJ). The place of contact between the terminal of a motor neuron and the membrane of a muscle fibre. Nerve impulses are transmitted across the gap by diffusion of a transmitter.
- MAJOR HISTOCOMPATIBILITY COMPLEX
-
(MHC). A cluster of genes on human chromosome 6 or mouse chromosome 17 that encode MHC molecules. These molecules are the most polymorphic in the genome and are the ones recognized by T lymphocytes during transplant rejection. They encode a family of cellular antigens that help the immune system to recognize self from non-self.
- SYNAPTIC CLEFT
-
The extracellular gap of a synapse that separates the outer membrane of the presynaptic nerve terminal from the postsynaptic membrane of the receiving cell.
- BASAL LAMINA
-
A thin sheet of extracellular matrix molecules, which consists mainly of collagens, laminins, nidogen and proteoglycans and forms a region between cells and adjacent connective tissue. It has an important influence on the organization of tissues.
- MYOTUBE
-
A multinucleated cell that is formed by the fusion of proliferating myoblasts and is characterized by the presence of certain muscle-specific marker proteins.
- SCHWANN CELL
-
A non-neuronal cell that produces myelin and ensheaths axons and presynaptic nerve terminals in the peripheral nervous system.
- MYOBLAST
-
An embryonic precursor cell that is still able to proliferate and can fuse to form myotubes.
- SUPERIOR CERVICAL GANGLION
-
(SCG). An aggregate of neurons of the sympathetic nervous system that are innervated by preganglionic neurons of the spinal cord. Postganglionic targets are the blood vessels, glands and pilomotor muscles of the head and neck.
- CREB
-
(cyclic AMP response-element-binding protein). A transcription factor that functions in glucose homeostasis and growth-factor-dependent cell survival, and has also been implicated in learning and memory.
- C-FOS
-
An immediate-early gene that belongs to the Fos family, which includes fosB, fra-1 and fra-2. These proteins form heterodimers with members of the Jun family and form transcription factors that bind to the AP-1 DNA element. Fos–Jun complexes have been implicated in many diverse cellular responses including learning and memory in the brain.
- MICROVASCULATURE
-
Very small blood vessels. In the brain, these fine cerebral vessels and capillaries maintain the blood–brain barrier and are the point of exchange for nutrients, electrolytes and metabolites between neural tissue and blood.
- AMYLOID PLAQUE
-
A deposition of amyloid β peptide in the brain that is a hallmark of Alzheimer's disease, a neurodegenerative disease that affects the elderly with a primary symptom of dementia.
- DENDRITIC ARBORIZATION
-
The ability of neurons to form an elaborate, arbor-like network of dendrites (receiving part of a neuron).
- SRC FAMILY KINASES
-
(SFKs). Kinases that belong to the Src family of tyrosine kinases, the largest of the non-receptor tyrosine kinases families. SFKs include Src, Yes, Fyn, Lck, Lyn, Blk, Hck, Fgr and Yrk.
- NEURAMINIDASE
-
An enzyme that catalyses the cleavage of neuraminic acid from oligosaccharide chains of glycoproteins and glycolipids. As these residues are usually terminal, neuraminidases are generally exo-enzymes, although an endoneuraminidase is known.
- GPI-ANCHOR
-
The function of this post-translational glycosylphosphatidylinositol modification is to attach proteins to the exoplasmic leaflet of membranes, possibly to specific domains therein. The anchor is made of one molecule of phosphatidylinositol to which a carbohydrate chain is linked through the C-6 hydroxyl of the inositol, and is linked to the protein through an ethanolamine phosphate moiety.
- PLANAR-CELL POLARITY
-
The polarization of cells in the plane of the epithelium. This phenomenon is best studied in Drosophila but is also found in vertebrates where it affects aspects of skin development and features of inner-ear development.
- RHO FAMILY GTPASES
-
Ras-related GTPases that are involved in controlling the polymerization of actin.
- DOMINANT-NEGATIVE
-
A defective protein that retains interaction abilities and so distorts or competes with normal proteins.
- RUFFLE
-
A cytoplasmic projection at the leading edge of a migrating cell that has lifted from the substratum. Molecules that are involved in actin cytoskeletal rearrangement are enriched in ruffles.
- MICROVILLUS
-
A small, finger-like projection (1–2 μm long and 100 nm wide) that occurs on the exposed surfaces of epithelial cells to maximize the surface area.
- FILOPODIUM
-
A finger-like exploratory cell extension that is found in crawling cells and growth cones.
- COSTAMERE
-
A myofibril attachment site that forms one of the main linkage sites of the skeletal muscle cell, encircling the cell and connecting the Z-discs to the sarcolemma.
- INTEGRINS
-
A large group of heterodimeric transmembrane adhesion receptors for several extracellular matrix proteins such as fibronectin, vitronectin and laminin.
- TRAILING EDGE
-
The region of the cell with a high concentration of adhesion receptors, ezrin–radixin–moesin (ERM) proteins, myosin II, tubulin cytoskeleton and protein kinase C, that has a pivotal role in cell adhesion and facilitates cell migration.
- LEADING EDGE
-
The thin margin of a lamellipodium spanning the area of the cell from the plasma membrane to about 1 μm back into the lamellipodium.
Rights and permissions
About this article
Cite this article
Bezakova, G., Ruegg, M. New insights into the roles of agrin. Nat Rev Mol Cell Biol 4, 295–309 (2003). https://doi.org/10.1038/nrm1074
Issue Date:
DOI: https://doi.org/10.1038/nrm1074
This article is cited by
-
A link between agrin signalling and Cav3.2 at the neuromuscular junction in spinal muscular atrophy
Scientific Reports (2022)
-
Deconstruction of Neurotrypsin Reveals a Multi-factorially Regulated Activity Affecting Myotube Formation and Neuronal Excitability
Molecular Neurobiology (2022)
-
Defective perlecan-associated basement membrane regeneration and altered modulation of transforming growth factor beta in corneal fibrosis
Cellular and Molecular Life Sciences (2022)
-
Functional significance of gain-of-function H19 lncRNA in skeletal muscle differentiation and anti-obesity effects
Genome Medicine (2021)
-
Basal lamina changes in neurodegenerative disorders
Molecular Neurodegeneration (2021)