Key Points
-
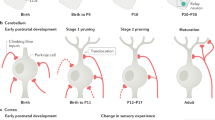
Brain development is associated with the formation of excessive synapses that have to be removed in a controlled and timely manner to achieve refined mature circuitry.
-
Glial cells, including microglia and astrocytes, are the effectors of synaptic pruning, identifying and eliminating superfluous synapses.
-
Synaptic pruning depends on various molecules, including those controlling glial chemotaxis, target recognition and phagocytosis.
-
Autism spectrum disorders are associated with excessive synapses, autophagy and dysregulated microglial function.
-
Schizophrenia is linked to exaggerated synaptic pruning owing to elevated levels of complement proteins and microglial activation.
-
Epilepsy is thought to arise owing to immature circuitry that was not refined via synaptic pruning. This initial epileptiform activity is followed by microglial activation and upregulation of complement components.
Abstract
The final stage of brain development is associated with the generation and maturation of neuronal synapses. However, the same period is also associated with a peak in synapse elimination — a process known as synaptic pruning — that has been proposed to be crucial for the maturation of remaining synaptic connections. Recent studies have pointed to a key role for glial cells in synaptic pruning in various parts of the nervous system and have identified a set of critical signalling pathways between glia and neurons. At the same time, brain imaging and post-mortem anatomical studies suggest that insufficient or excessive synaptic pruning may underlie several neurodevelopmental disorders, including autism, schizophrenia and epilepsy. Here, we review current data on the cellular, physiological and molecular mechanisms of glial-cell-dependent synaptic pruning and outline their potential contribution to neurodevelopmental disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Riccomagno, M. M. & Kolodkin, A. L. Sculpting neural circuits by axon and dendrite pruning. Annu. Rev. Cell Dev. Biol. 31, 779–805 (2015).
Innocenti, G. M. & Price, D. J. Exuberance in the development of cortical networks. Nat. Rev. Neurosci. 6, 955–965 (2005).
Johnson, M. H. Functional brain development in humans. Nat. Rev. Neurosci. 2, 475–483 (2001).
Darabid, H., Perez-Gonzalez, A. P. & Robitaille, R. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat. Rev. Neurosci. 15, 703–718 (2014).
Hashimoto, K. & Kano, M. Synapse elimination in the developing cerebellum. Cell. Mol. Life Sci. 70, 4667–4680 (2013).
Huberman, A. D. Mechanisms of eye-specific visual circuit development. Curr. Opin. Neurobiol. 17, 73–80 (2007).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).This was the first study to demonstrate that microglial cells are required for synaptic pruning.
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).This study presented the first molecular mechanism by which microglia prune superfluous synapses.
Chung, W. S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013).This study introduced astrocytes as cells capable of synaptic pruning and described astrocytic receptors involved in the process.
Chung, W. S. et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc. Natl Acad. Sci. USA (2016).
Sipe, G. O. et al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 7, 10905 (2016).
Schafer, D. P. & Stevens, B. Microglia function in central nervous system development and plasticity. Cold Spring Harb. Perspect. Biol. 7, a020545 (2015).
Clarke, L. E. & Barres, B. A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321 (2013).
Darabid, H., Arbour, D. & Robitaille, R. Glial cells decipher synaptic competition at the mammalian neuromuscular junction. J. Neurosci. 33, 1297–1313 (2013).
Berbel, P. & Innocenti, G. M. The development of the corpus callosum in cats: a light- and electron-microscopic study. J. Comp. Neurol. 276, 132–156 (1988).This classic study was the first to indicate that glial cells are involved in synaptic pruning.
Hoshiko, M., Arnoux, I., Avignone, E., Yamamoto, N. & Audinat, E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J. Neurosci. 32, 15106–15111 (2012).
Zhan, Y. et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Ichikawa, R. et al. Developmental switching of perisomatic innervation from climbing fibers to basket cell fibers in cerebellar Purkinje cells. J. Neurosci. 31, 16916–16927 (2011).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).This study described upregulated C4 as a risk factor for schizophrenia, linking aberrant synaptic pruning to the pathology of the disease.
Blakemore, S. J. Development of the social brain during adolescence. Q. J. Exp. Psychol. (Hove) 61, 40–49 (2008).
Smith, I. W., Mikesh, M., Lee, Y. & Thompson, W. J. Terminal Schwann cells participate in the competition underlying neuromuscular synapse elimination. J. Neurosci. 33, 17724–17736 (2013).
Tapia, J. C. et al. Pervasive synaptic branch removal in the mammalian neuromuscular system at birth. Neuron 74, 816–829 (2012).
Bishop, D. L., Misgeld, T., Walsh, M. K., Gan, W. B. & Lichtman, J. W. Axon branch removal at developing synapses by axosome shedding. Neuron 44, 651–661 (2004).
Song, J. W. et al. Lysosomal activity associated with developmental axon pruning. J. Neurosci. 28, 8993–9001 (2008).
Lee, Y. I. et al. Neuregulin1 displayed on motor axons regulates terminal Schwann cell-mediated synapse elimination at developing neuromuscular junctions. Proc. Natl Acad. Sci. USA 113, E479–E487 (2016).
Wang, J. Y. et al. Caspase-3 cleavage of dishevelled induces elimination of postsynaptic structures. Dev. Cell 28, 670–684 (2014).
Todd, K. J., Darabid, H. & Robitaille, R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J. Neurosci. 30, 11870–11882 (2010).
Carrillo, J., Nishiyama, N. & Nishiyama, H. Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination. J. Neurosci. 33, 7641–7653 (2013).
Hashimoto, K. & Kano, M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron 38, 785–796 (2003).
Andjus, P. R., Zhu, L., Cesa, R., Carulli, D. & Strata, P. A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum. Neuroscience 121, 563–572 (2003).
Sugihara, I. Microzonal projection and climbing fiber remodeling in single olivocerebellar axons of newborn rats at postnatal days 4–7. J. Comp. Neurol. 487, 93–106 (2005).
Iino, M. et al. Glia–synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929 (2001).
Kakegawa, W. et al. Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85, 316–329 (2015).
Ballesteros, J. M., Van Der List, D. A. & Chalupa, L. M. Formation of eye-specific retinogeniculate projections occurs prior to the innervation of the dorsal lateral geniculate nucleus by cholinergic fibers. Thalamus Relat. Syst. 3, 157–163 (2005).
Hong, Y. K. et al. Refinement of the retinogeniculate synapse by bouton clustering. Neuron 84, 332–339 (2014).
Perry, V. H. & O'Connor, V. C1q: the perfect complement for a synaptic feast? Nat. Rev. Neurosci. 9, 807–811 (2008).
Hajishengallis, G. & Lambris, J. D. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 31, 154–163 (2010).
Le Cabec, V., Carreno, S., Moisand, A., Bordier, C. & Maridonneau-Parini, I. Complement receptor 3 (CD11b/CD18) mediates type I and type II phagocytosis during nonopsonic and opsonic phagocytosis, respectively. J. Immunol. 169, 2003–2009 (2002).
Linnartz, B., Kopatz, J., Tenner, A. J. & Neumann, H. Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor-3-mediated removal by microglia. J. Neurosci. 32, 946–952 (2012).
Schafer, D. P. et al. Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. Elife 5, e15224 (2016).This report linked microglial synaptic pruning to the progression of Rett syndrome.
Cahoy, J. D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Low, L. K., Liu, X. B., Faulkner, R. L., Coble, J. & Cheng, H. J. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc. Natl Acad. Sci. USA 105, 8136–8141 (2008).
Faulkner, R. L., Low, L. K. & Cheng, H. J. Axon pruning in the developing vertebrate hippocampus. Dev. Neurosci. 29, 6–13 (2007).
Tremblay, M. E., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010).This was the first study to present live microglial-cell–synapse interactions in the brain.
Espinosa, J. S. & Stryker, M. P. Development and plasticity of the primary visual cortex. Neuron 75, 230–249 (2012).
Sieger, D., Moritz, C., Ziegenhals, T., Prykhozhij, S. & Peri, F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev. Cell 22, 1138–1148 (2012).
Haynes, S. E. et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519 (2006).
Sasaki, T. et al. Developmental expression profiles of axon guidance signaling and the immune system in the marmoset cortex: potential molecular mechanisms of pruning of dendritic spines during primate synapse formation in late infancy and prepuberty (I). Biochem. Biophys. Res. Commun. 444, 302–306 (2014).
Sasaki, T. et al. Developmental genetic profiles of glutamate receptor system, neuromodulator system, protector of normal tissue and mitochondria, and reelin in marmoset cortex: potential molecular mechanisms of pruning phase of spines in primate synaptic formation process during the end of infancy and prepuberty (II). Biochem. Biophys. Res. Commun. 444, 307–310 (2014).
LaMantia, A. S. & Rakic, P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J. Neurosci. 10, 2156–2175 (1990).
Bourgeois, J. P., Goldman-Rakic, P. S. & Rakic, P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex 4, 78–96 (1994).
Perry, V. H., Hume, D. A. & Gordon, S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15, 313–326 (1985).
Mody, M. et al. Genome-wide gene expression profiles of the developing mouse hippocampus. Proc. Natl Acad. Sci. USA 98, 8862–8867 (2001).
Block, M., Zecca, L. & Hong, J. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 (2007).
van Loo, K. M. & Martens, G. J. Genetic and environmental factors in complex neurodevelopmental disorders. Curr. Genomics 8, 429–444 (2007).
Pierce, K. Early functional brain development in autism and the promise of sleep fMRI. Brain Res. 1380, 162–174 (2011).
Sacco, R., Gabriele, S. & Persico, A. M. Head circumference and brain size in autism spectrum disorder: a systematic review and meta-analysis. Psychiatry Res. 234, 239–251 (2015).
Redcay, E. & Courchesne, E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol. Psychiatry 58, 1–9 (2005).
Suzuki, K. et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 70, 49–58 (2013).
Dinstein, I., Haar, S., Atsmon, S. & Schtaerman, H. No evidence of early head circumference enlargements in children later diagnosed with autism in Israel. Mol. Autism 8, 15 (2017).
Raznahan, A. et al. Compared to what? Early brain overgrowth in autism and the perils of population norms. Biol. Psychiatry 74, 563–575 (2013).
Lewis, J. D., Theilmann, R. J., Townsend, J. & Evans, A. C. Network efficiency in autism spectrum disorder and its relation to brain overgrowth. Front. Hum. Neurosci. 7, 845 (2013).
Lewis, J. D. et al. Callosal fiber length and interhemispheric connectivity in adults with autism: brain overgrowth and underconnectivity. Hum. Brain Mapp. 34, 1685–1695 (2013).
Dinstein, I. et al. Disrupted neural synchronization in toddlers with autism. Neuron 70, 1218–1225 (2011).
Dichter, G. S. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin. Neurosci. 14, 319–351 (2012).
Barttfeld, P. et al. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia 49, 254–263 (2011).
Tang, G. et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83, 1131–1143 (2014).This study revealed excessive synapses in autistic brains and presented a druggable target that is involved in synaptic pruning.
Hutsler, J. J. & Zhang, H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 1309, 83–94 (2010).
Piochon, C. et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 5, 5586 (2014).
Kim, H. J. et al. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry https://dx.doi.org/10.1038/mp.2016.103 (2016).
Voineagu, I. et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384 (2011).
Nardone, S. et al. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl Psychiatry 4, e433 (2014).
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014).
Miyazaki, S., Hiraoka, Y., Hidema, S. & Nishimori, K. Prenatal minocycline treatment alters synaptic protein expression, and rescues reduced mother call rate in oxytocin receptor-knockout mice. Biochem. Biophys. Res. Commun. 472, 319–323 (2016).
Selemon, L. D. & Zecevic, N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry 5, e623 (2015).
Casey, B. J., Jones, R. M. & Hare, T. A. The adolescent brain. Ann. NY Acad. Sci. 1124, 111–126 (2008).
Zhang, Y. et al. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry 6, e982 (2016).
Tomasi, D. & Volkow, N. D. Mapping small-world properties through development in the human brain: disruption in schizophrenia. PLoS ONE 9, e96176 (2014).
Alexander-Bloch, A. F. et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb. Cortex 23, 127–138 (2013).
Glantz, L. A. & Lewis, D. A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 57, 65–73 (2000).
Kolluri, N., Sun, Z., Sampson, A. R. & Lewis, D. A. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry 162, 1200–1202 (2005).
Rakic, P., Bourgeois, J. P., Eckenhoff, M. F., Zecevic, N. & Goldman-Rakic, P. S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 232, 232–235 (1986).This classical study revealed that brain development is associated with the formation of superfluous excitatory synapses that are subsequently eliminated.
Bourgeois, J. P. & Rakic, P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. 13, 2801–2820 (1993).
Cocchi, E., Drago, A. & Serretti, A. Hippocampal pruning as a new theory of schizophrenia etiopathogenesis. Mol. Neurobiol. 53, 2065–2081 (2016).
Calabro, M., Drago, A., Sidoti, A., Serretti, A. & Crisafulli, C. Genes involved in pruning and inflammation are enriched in a large mega-sample of patients affected by schizophrenia and bipolar disorder and controls. Psychiatry Res. 228, 945–949 (2015).
Bayer, T. A., Buslei, R., Havas, L. & Falkai, P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci. Lett. 271, 126–128 (1999).
Doorduin, J. et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J. Nucl. Med. 50, 1801–1807 (2009).
Inta, D., Lang, U. E., Borgwardt, S., Meyer-Lindenberg, A. & Gass, P. Microglia activation and schizophrenia: lessons from the effects of minocycline on postnatal neurogenesis, neuronal survival and synaptic pruning. Schizophr. Bull. 43, 493–496 (2016).
Mayilyan, K. R., Weinberger, D. R. & Sim, R. B. The complement system in schizophrenia. Drug News Perspect. 21, 200–210 (2008).
Mayilyan, K. R., Dodds, A. W., Boyajyan, A. S., Soghoyan, A. F. & Sim, R. B. Complement C4B protein in schizophrenia. World J. Biol. Psychiatry 9, 225–230 (2008).
Severance, E. G., Gressitt, K. L., Buka, S. L., Cannon, T. D. & Yolken, R. H. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr. Res. 159, 14–19 (2014).
Fananas, L., Moral, P., Panadero, M. A. & Bertranpetit, J. Complement genetic markers in schizophrenia: C3, BF and C6 polymorphisms. Hum. Hered. 42, 162–167 (1992).
Myers, C. T. & Mefford, H. C. Advancing epilepsy genetics in the genomic era. Genome Med. 7, 91 (2015).
Eyo, U. B., Murugan, M. & Wu, L. J. Microglia–neuron communication in epilepsy. Glia 65, 5–18 (2016).
Zhou, Y. D. et al. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat. Med. 15, 1208–1214 (2009).
Zhou, Y. D. et al. Epilepsy gene LGI1 regulates postnatal developmental remodeling of retinogeniculate synapses. J. Neurosci. 32, 903–910 (2012).
van Campen, J. S. et al. Sensory modulation disorders in childhood epilepsy. J. Neurodev Disord. 7, 34 (2015).
Head, K. et al. Defining the expression pattern of the LGI1 gene in BAC transgenic mice. Mamm. Genome 18, 328–337 (2007).
Chu, Y. et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl Acad. Sci. USA 107, 7975–7980 (2010).This study demonstrated that impairment of developmental synaptic pruning leads to an epileptic phenotype.
Ma, Y., Ramachandran, A., Ford, N., Parada, I. & Prince, D. A. Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy. Epilepsia 54, 1232–1239 (2013).
Aronica, E. et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol. Dis. 26, 497–511 (2007).
Xu, Y. et al. Altered expression of CX3CL1 in patients with epilepsy and in a rat model. Am. J. Pathol. 180, 1950–1962 (2012).
Roseti, C. et al. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia 54, 1834–1844 (2013).
Ali, I., Chugh, D. & Ekdahl, C. T. Role of fractalkine–CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain. Neurobiol. Dis. 74, 194–203 (2015).
Neher, J. J., Neniskyte, U. & Brown, G. C. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front. Pharmacol. 3, 27 (2012).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009).
Tremblay, M. E., Zettel, M. L., Ison, J. R., Allen, P. D. & Majewska, A. K. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia 60, 541–558 (2012).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Arnoux, I. & Audinat, E. Fractalkine signaling and microglia functions in the developing brain. Neural Plast. http://dx.doi.org/10.1155/2015/689404 (2015).
Erturk, A., Wang, Y. & Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 34, 1672–1688 (2014).
Awasaki, T. & Ito, K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr. Biol. 14, 668–677 (2004).
Marin, E. C., Watts, R. J., Tanaka, N. K., Ito, K. & Luo, L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development 132, 725–737 (2005).
Tasdemir-Yilmaz, O. E. & Freeman, M. R. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 28, 20–33 (2014).
Awasaki, T. et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50, 855–867 (2006).
Milior, G. et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav. Immun. 55, 114–125 (2016).
Spiga, S. et al. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc. Natl Acad. Sci. USA 111, E3745–E3754 (2014).
Shi, Q. et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci. 35, 13029–13042 (2015).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Vasek, M. J. et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 (2016).
Iram, T. et al. Megf10 is a receptor for C1Q that mediates clearance of apoptotic cells by astrocytes. J. Neurosci. 36, 5185–5192 (2016).
Beisiegel, U., Weber, W., Ihrke, G., Herz, J. & Stanley, K. K. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature 341, 162–164 (1989).
Acknowledgements
U.N. has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 705452, International Brain Research Organization Return Home Fellowship, and L'ORÉAL Baltic “For Women In Science” fellowship with the support of the Lithuanian National Commission for the United Nations Educational, Scientific and Cultural Organization (UNESCO) and the Lithuanian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
U.N. and C.T.G. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Neuromuscular junction
-
(NMJ). A peripheral synapse between one or more motor neurons and the motor endplate on a skeletal muscle fibre.
- Receptive fields
-
Particular regions of a sensory space, such as the visual field, in which a stimulus will modify the firing of an individual sensory neuron.
- Topographic mappings
-
Ordered projections of a sensory surface, such as the retina, to one or more structures of the central nervous system (such as the lateral geniculate nucleus and the visual cortex).
- Climbing fibres
-
Axons of inferior olivary neurons that form excitatory synapses with Purkinje cells in the cerebellum.
- Bergmann glia
-
Radial astrocytes in the cerebellar cortex that are involved in early cerebellar development, glutamate diffusion control, synaptogenesis and synaptic pruning.
- Minocycline
-
A tetracycline antibiotic that has been shown to inhibit inflammatory activation of microglia by blocking the nuclear translocation of the pro-inflammatory transcription factor nuclear factor-κB.
- Retinal waves
-
Spontaneous bursts of action potentials that propagate in a wave-like fashion across the developing retina.
- Apolipoprotein E
-
(APOE). A major cholesterol carrier that is also hypothesized to serve as an opsonin. The APOE*4 allele is a major genetic risk factor for Alzheimer disease.
- Opsonin
-
A protein, such as an antibody or complement protein, that binds to a phagocytic target (such as a pathogen), thus rendering it more susceptible to phagocytosis, in a process known as opsonization.
- Archicortex
-
A phylogenetically old part of the cerebral cortex that constitutes the hippocampal formation.
- Synaptic multiplicity
-
A feature of mature circuits in which afferent inputs make more than one synapse onto a single target neuron.
- Window-on-the-brain technology
-
A technique in which the skull is thinned or opened and capped with a transparent implant to allow two-photon or near-infrared in vivo imaging of cortical function.
- Autism spectrum disorder
-
(ASD). A group of neurodevelopmental conditions characterized by social deficits, impaired language development, intellectual disability, increased repetitive or restrictive behaviours and motor abnormalities.
- Peripheral benzodiazepine receptor
-
Translocator protein (TSPO) of the outer mitochondrial membrane that modulates bursts of reactive oxygen species in macrophages, including microglia, and is therefore used as a marker of inflammation.
- Penetrant
-
Of a mutation, producing expression of associated phenotypic traits in a large proportion of individuals carrying the mutation.
- Autophagy
-
An intracellular self-degradative process for orderly degradation and recycling of cellular components and balancing sources of energy at critical periods.
- Microgliosis
-
An intense inflammatory activation of microglia in response to insults to the CNS (such as infection, trauma or neuronal damage).
- Ocular dominance index
-
The difference between contralateral response and ipsilateral response divided by the sum of contralateral and ipsilateral responses.
Rights and permissions
About this article
Cite this article
Neniskyte, U., Gross, C. Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci 18, 658–670 (2017). https://doi.org/10.1038/nrn.2017.110
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.110
This article is cited by
-
Single nuclei transcriptomics in human and non-human primate striatum in opioid use disorder
Nature Communications (2024)
-
Neuroanatomical dimensions in medication-free individuals with major depressive disorder and treatment response to SSRI antidepressant medications or placebo
Nature Mental Health (2024)
-
Prolonged anesthesia induces neuroinflammation and complement-mediated microglial synaptic elimination involved in neurocognitive dysfunction and anxiety-like behaviors
BMC Medicine (2023)
-
Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis
Journal of Neuroinflammation (2023)
-
Sexual dimorphism in the social behaviour of Cntnap2-null mice correlates with disrupted synaptic connectivity and increased microglial activity in the anterior cingulate cortex
Communications Biology (2023)