Key Points
-
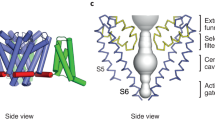
The gate region in K+ channels controls whether ions can traverse the ion conduction pore. Studies using quaternary ammonium compounds and the X-ray structure of the KcsA K+ channels are consistent with the presence of a gate at the intracellular side of the pore.
-
There is substantial evidence that the structure of the gate region of Kv channels and the movements that occur during opening are different from that proposed for KcsA and MthK.
-
The S4 transmembrane segment of the channels is rich in positively charged basic residues, and S4 is agreed to be important in voltage sensing, although the conceptual and structural basis for gating charge movement is the subject of intense controversy.
-
There are two models for the movement of gating charges across the channels. The 'membrane translocation model' proposes that the gating charges completely translocate from one side of the hydrophobic phase of the membrane to the other, which corresponds to a movement of more than 20 Å. By contrast, the 'focused field model' proposes that charges move shorter distances between water-filled crevices in the protein, which serve to focus the electric field of the membrane.
-
Most studies favoured the focused field model until 2003 when the X-ray structure of the KvAP channel was revealed. The X-ray structure of the intact KvAP protein was solved to 3.2 Å resolution, using Fab fragments of monoclonal antibodies bound to the voltage-sensors. This provided important evidence, which is in line with the translocation model but contradictory to the focused field model.
-
Although MacKinnon and colleagues pointed out several reasons for suggesting that there could be distortions in the X-ray structure of KvAP, they concluded that the crystallized full-length channel is not far from a membrane-bound conformation.
-
At present, the extent to which the structure of KvAP is distorted remains a central issue in the debate. This review argues that there are many indications that the distortions are extensive.
-
The original paddle model for gating charge movement is a membrane translocation model that is supported by an intuitive interpretation of how and why the KvAP structure is distorted, and by functional experiments and electron microscopy reconstruction.
-
Unlike the X-ray structure of KvAP, the electron microscopy reconstruction of KvAP is compatible with the focused field model because the S3 and S4 segments have transmembrane orientations. The focused field model is also supported by experiments demonstrating that S4 can form a proton conducting channel, and by experiments showing that the C terminus of S3 and the N terminus of S4 are positioned near the extracellular side of the membrane in the resting conformation.
-
Although the membrane translocation and focused field models are useful for considering the mechanisms that underlie voltage-sensing, neither model has a solid structural framework. Future work should focus on the tertiary structure of the voltage sensing domain and the spatial proximity of specific residues within well-defined secondary structural elements of the voltage-sensors.
Abstract
Voltage-activated cation channels have pores that are selective for K+, Na+ or Ca2+. Neurons use these channels to generate and propagate action potentials, release neurotransmitters at synaptic terminals and integrate incoming signals in dendrites. Recent X-ray and electron microscopy studies of an archaebacterial voltage-activated K+ (Kv) channel have provided the first atomic resolution images of the voltage-sensing domains in Kv channels. Although these structures are consistent with previous biophysical analyses of eukaryotic channels, they also contain surprises, which have provoked new ideas about the structure and movements of these proteins during gating. This review summarizes our current understanding of these intriguing membrane proteins and highlights the open questions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Hodgkin, A. L. & Huxley, A. F. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 116, 473–496 (1952).
Hodgkin, A. L. & Huxley, A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 116, 449–472 (1952).
Hodgkin, A. L. & Huxley, A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J. Physiol. 116, 497–506 (1952).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Papazian, D. M., Schwarz, T. L., Tempel, B. L., Jan, Y. N. & Jan, L. Y. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science 237, 749–753 (1987).
Timpe, L. C. et al. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature 331, 143–145 (1988).
Zagotta, W. N., Hoshi, T. & Aldrich, R. W. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 103, 321–362 (1994).
Zagotta, W. N., Hoshi, T., Dittman, J. & Aldrich, R. W. Shaker potassium channel gating. II: Transitions in the activation pathway. J. Gen. Physiol. 103, 279–319 (1994).
Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Shaker potassium channel gating. I: Transitions near the open state. J. Gen. Physiol. 103, 249–278 (1994).
Schoppa, N. E. & Sigworth, F. J. Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111, 313–342 (1998).
Schoppa, N. E. & Sigworth, F. J. Activation of Shaker potassium channels. II. Kinetics of the V2 mutant channel. J. Gen. Physiol. 111, 295–311 (1998).
Schoppa, N. E. & Sigworth, F. J. Activation of Shaker potassium channels. I. Characterization of voltage-dependent transitions. J. Gen. Physiol. 111, 271–294 (1998).
Islas, L. D. & Sigworth, F. J. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J. Gen. Physiol. 114, 723–741 (1999).
Soler-Llavina, G. J., Holmgren, M. & Swartz, K. J. Defining the conductance of the closed state in a voltage-gated K+ channel. Neuron 38, 61–67 (2003).
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998). The first X-ray structure of a potassium channel. The KcsA potassium channel is a prokaryotic channel from Streptomyces lividans that was crystallized in a closed conformation.
Zhou, Y., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001).
Zhou, M., Morais-Cabral, J. H., Mann, S. & MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature 411, 657–661 (2001).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002). The first X-ray structure of a potassium channel in the open conformation. This paper also shows the octameric arrangement of the RCK domains that form a gating ring on the intracellular side of the channel. A detailed discussion of gating motions is presented in the companion paper, reference 60.
Kuo, A. et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926 (2003).
Jiang, Y. et al. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41 (2003). The first X-ray structure of a voltage-activated potassium channel. This paper reports two structures for KvAP — one for the intact channel protein and another for the isolated S1–S4 voltage-sensing domain.
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002).
Ren, D. et al. A prokaryotic voltage-gated sodium channel. Science 294, 2372–2375 (2001).
Koishi, R. et al. A superfamily of voltage-gated sodium channels in bacteria. J. Biol. Chem. 279, 9532–9538 (2004).
Noda, M. et al. Expression of functional sodium channels from cloned cDNA. Nature 322, 826–828 (1986).
Noda, M. et al. Existence of distinct sodium channel messenger RNAs in rat brain. Nature 320, 188–192 (1986).
Tanabe, T. et al. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 328, 313–318 (1987).
Goulding, E. H. et al. Molecular cloning and single-channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron 8, 45–58 (1992).
Santoro, B. et al. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93, 717–729 (1998).
Butler, A., Tsunoda, S., McCobb, D. P., Wei, A. & Salkoff, L. mSlo, a complex mouse gene encoding 'maxi' calcium-activated potassium channels. Science 261, 221–224 (1993).
Kubo, Y., Reuveny, E., Slesinger, P. A., Jan, Y. N. & Jan, L. Y. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364, 802–806 (1993).
Kubo, Y., Baldwin, T. J., Jan, Y. N. & Jan, L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362, 127–133 (1993).
Ho, K. et al. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362, 31–38 (1993).
Ketchum, K. A., Joiner, W. J., Sellers, A. J., Kaczmarek, L. K. & Goldstein, S. A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 376, 690–695 (1995).
Goldstein, S. A., Bockenhauer, D., O'Kelly, I. & Zilberberg, N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nature Rev. Neurosci. 2, 175–184 (2001).
Chen, G. Q., Cui, C., Mayer, M. L. & Gouaux, E. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402, 817–821 (1999).
MacKinnon, R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350, 232–235 (1991).
Liman, E. R., Tytgat, J. & Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 (1992).
Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990).
Zagotta, W. N., Hoshi, T. & Aldrich, R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science 250, 568–571 (1990).
Santacruz-Toloza, L., Huang, Y., John, S. A. & Papazian, D. M. Glycosylation of Shaker potassium channel protein in insect cell culture and in Xenopus oocytes. Biochemistry 33, 5607–5613 (1994).
Holmgren, M., Jurman, M. E. & Yellen, G. N-type inactivation and the S4-S5 region of the Shaker K+ channel. J. Gen. Physiol. 108, 195–206 (1996).
Li-Smerin, Y. & Swartz, K. J. Localization and molecular determinants of the hanatoxin receptors on the voltage-sensing domain of a K+ channel. J. Gen. Physiol. 115, 673–684 (2000).
Li-Smerin, Y. & Swartz, K. J. Helical structure of the COOH terminus of S3 and its contribution to the gating modifier toxin receptor in voltage-gated ion channels. J. Gen. Physiol. 117, 205–218 (2001).
Lee, H. C., Wang, J. M. & Swartz, K. J. Interaction between extracellular Hanatoxin and the resting conformation of the voltage-sensor paddle in Kv channels. Neuron 40, 527–536 (2003). This paper investigates the effects of a protein toxin from tarantula venom on gating charge movement, which provides evidence for a relatively extracellular position for the S3b helix when the voltage sensors are in their resting conformations.
Yang, N., George, A. L., Jr & Horn, R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron 16, 113–122 (1996).
Larsson, H. P., Baker, O. S., Dhillon, D. S. & Isacoff, E. Y. Transmembrane movement of the Shaker K+ channel S4. Neuron 16, 387–397 (1996).
Yusaf, S. P., Wray, D. & Sivaprasadarao, A. Measurement of the movement of the S4 segment during the activation of a voltage-gated potassium channel. Pflugers Arch. 433, 91–97 (1996).
MacKinnon, R. & Miller, C. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J. Gen. Physiol. 91, 335–349 (1988).
MacKinnon, R., Heginbotham, L. & Abramson, T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron 5, 767–771 (1990).
Armstrong, C. M. Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J. Gen. Physiol. 54, 553–575 (1969).
Armstrong, C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58, 413–437 (1971).
Armstrong, C. M. Ionic pores, gates, and gating currents. Q. Rev. Biophys. 7, 179–210 (1974). An intelligent review that lays out a conceptual framework for understanding the mechanisms that underlie voltage-dependent gating and cation selectivity. This review was years ahead of its time and is a true classic in the field.
Holmgren, M., Smith, P. L. & Yellen, G. Trapping of organic blockers by closing of voltage-dependent K+ channels: evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109, 527–535 (1997). This paper clearly shows the trapping of quaternary ammonium compounds in the intracellular pore of the Shaker potassium channel.
Liu, Y., Holmgren, M., Jurman, M. E. & Yellen, G. Gated access to the pore of a voltage-dependent K+ channel. Neuron 19, 175–184 (1997). A classic paper that shows the gated access of MTS reagents for Cys residues substituted in the intracellular region of the S6 segment in the Shaker potassium channel.
del Camino, D. & Yellen, G. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron 32, 649–656 (2001).
Holmgren, M., Shin, K. S. & Yellen, G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron 21, 617–621 (1998).
Sukhareva, M., Hackos, D. H. & Swartz, K. J. Constitutive activation of the Shaker Kv channel. J. Gen. Physiol. 122, 541–556 (2003).
Kitaguchi, T., Sukhareva, M. & Swartz, K. J. Stabilizing the closed S6 gate in the Shaker Kv channel through modification of a hydrophobic seal. J. Gen. Physiol. 124, 319–332 (2004).
Roux, B., Berneche, S. & Im, W. Ion channels, permeation, and electrostatics: insight into the function of KcsA. Biochemistry 39, 13295–13306 (2000).
Jiang, Y. et al. The open pore conformation of potassium channels. Nature 417, 523–526 (2002).
Perozo, E., Cortes, D. M. & Cuello, L. G. Structural rearrangements underlying K+-channel activation gating. Science 285, 73–78 (1999).
Liu, Y. S., Sompornpisut, P. & Perozo, E. Structure of the KcsA channel intracellular gate in the open state. Nature Struct. Biol. 8, 883–887 (2001).
Kelly, B. L. & Gross, A. Potassium channel gating observed with site-directed mass tagging. Nature Struct. Biol. 10, 280–284 (2003).
Irizarry, S. N., Kutluay, E., Drews, G., Hart, S. J. & Heginbotham, L. Opening the KcsA K+ channel: tryptophan scanning and complementation analysis lead to mutants with altered gating. Biochemistry 41, 13653–13662 (2002).
MacArthur, M. W. & Thornton, J. M. Influence of proline residues on protein conformation. J. Mol. Biol. 218, 397–412 (1991).
Del Camino, D., Holmgren, M., Liu, Y. & Yellen, G. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature 403, 321–325 (2000).
Webster, S. M., Del Camino, D., Dekker, J. P. & Yellen, G. Intracellular gate opening in Shaker K+ channels defined by high-affinity metal bridges. Nature 428, 864–868 (2004). This paper investigates metal bridges in the gate region of the Shaker potassium channel that are not compatible with the X-ray structures of either KcsA, which was crystallized in a closed conformation, or MthK, which was crystallized in an open conformation.
Armstrong, C. M. & Bezanilla, F. Currents related to movement of the gating particles of the sodium channels. Nature 242, 459–461 (1973).
Armstrong, C. M. & Bezanilla, F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63, 533–552 (1974).
Keynes, R. D. & Rojas, E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J. Physiol. 239, 393–434 (1974).
Schneider, M. F. & Chandler, W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature 242, 244–246 (1973).
Chandler, W. K., Schneider, M. F., Rakowski, R. F. & Adrian, R. H. Charge movements in skeletal muscle. Phil. Trans. R. Soc. Lond. B 270, 501–505 (1975).
Bezanilla, F. & Stefani, E. Gating currents. Methods Enzymol. 293, 331–352 (1998).
Schoppa, N. E., McCormack, K., Tanouye, M. A. & Sigworth, F. J. The size of gating charge in wild-type and mutant Shaker potassium channels. Science 255, 1712–1715 (1992).
Aggarwal, S. K. & MacKinnon, R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 16, 1169–1177 (1996).
Seoh, S. A., Sigg, D., Papazian, D. M. & Bezanilla, F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 16, 1159–1167 (1996).
Noceti, F. et al. Effective gating charges per channel in voltage-dependent K+ and Ca2+ channels. J. Gen. Physiol. 108, 143–155 (1996).
Hirschberg, B., Rovner, A., Lieberman, M. & Patlak, J. Transfer of twelve charges is needed to open skeletal muscle Na+ channels. J. Gen. Physiol. 106, 1053–1068 (1995).
Auld, V. J. et al. A neutral amino acid change in segment IIS4 dramatically alters the gating properties of the voltage-dependent sodium channel. Proc. Natl Acad. Sci. USA 87, 323–327 (1990).
Papazian, D. M., Timpe, L. C., Jan, Y. N. & Jan, L. Y. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature 349, 305–310 (1991).
Liman, E. R., Hess, P., Weaver, F. & Koren, G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature 353, 752–756 (1991).
Smith-Maxwell, C. J., Ledwell, J. L. & Aldrich, R. W. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J. Gen. Physiol. 111, 421–439 (1998).
Smith-Maxwell, C. J., Ledwell, J. L. & Aldrich, R. W. Role of the S4 in cooperativity of voltage-dependent potassium channel activation. J. Gen. Physiol. 111, 399–420 (1998).
Ledwell, J. L. & Aldrich, R. W. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 113, 389–414 (1999).
Perozo, E., Santacruz-Toloza, L., Stefani, E., Bezanilla, F. & Papazian, D. M. S4 mutations alter gating currents of Shaker K channels. Biophys. J. 66, 345–354 (1994).
Ahern, C. A. & Horn, R. Specificity of charge-carrying residues in the voltage sensor of potassium channels. J. Gen. Physiol. 123, 205–216 (2004). A recent paper that examines where in S4 the addition of charged MTS moieties contributes to the total gating charge per channel. The results indicate that only the positions that are charged in the wild-type channel are capable of contributing to gating charge.
Yang, N. & Horn, R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron 15, 213–218 (1995). A classic study that investigated the movements of S4 by measuring the voltage-dependence of the reaction between MTS reagents and Cys residues substituted in S4.
Starace, D. M., Stefani, E. & Bezanilla, F. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron 19, 1319–1327 (1997).
Starace, D. M. & Bezanilla, F. Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J. Gen. Physiol. 117, 469–490 (2001).
Mannuzzu, L. M., Moronne, M. M. & Isacoff, E. Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science 271, 213–216 (1996). This was the first study that investigated S4 movements using fluorescence spectroscopy.
Cha, A. & Bezanilla, F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron 19, 1127–1140 (1997).
Cha, A. & Bezanilla, F. Structural implications of fluorescence quenching in the Shaker K+ channel. J. Gen. Physiol. 112, 391–408 (1998).
Glauner, K. S., Mannuzzu, L. M., Gandhi, C. S. & Isacoff, E. Y. Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel. Nature 402, 813–817 (1999).
Cha, A., Snyder, G. E., Selvin, P. R. & Bezanilla, F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature 402, 809–813 (1999).
Gandhi, C. S., Loots, E. & Isacoff, E. Y. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron 27, 585–595 (2000).
Papazian, D. M. et al. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron 14, 1293–1301 (1995).
Planells-Cases, R., Ferrer-Montiel, A. V., Patten, C. D. & Montal, M. Mutation of conserved negatively charged residues in the S2 and S3 transmembrane segments of a mammalian K+ channel selectively modulates channel gating. Proc. Natl Acad. Sci. USA 92, 9422–9426 (1995).
Tiwari-Woodruff, S. K., Schulteis, C. T., Mock, A. F. & Papazian, D. M. Electrostatic interactions between transmembrane segments mediate folding of Shaker K+ channel subunits. Biophys. J. 72, 1489–1500 (1997).
Li-Smerin, Y., Hackos, D. H. & Swartz, K. J. Alpha-helical structural elements within the voltage-sensing domains of a K+ channel. J. Gen. Physiol. 115, 33–50 (2000).
Nguyen, T. P. & Horn, R. Movement and crevices around a sodium channel S3 segment. J. Gen. Physiol. 120, 419–436 (2002).
Starace, D. M. & Bezanilla, F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427, 548–553 (2004). This paper shows the creation of a proton-conducting pore within the voltage-sensor of the Shaker potassium channel.
Bezanilla, F. Voltage sensor movements. J. Gen. Physiol. 120, 465–473 (2002). An excellent review of what is known about how S4 moves during gating in voltage-activated channels.
Horn, R. Coupled movements in voltage-gated ion channels. J. Gen. Physiol. 120, 449–453 (2002).
Gandhi, C. S. & Isacoff, E. Y. Molecular models of voltage sensing. J. Gen. Physiol. 120, 455–463 (2002).
Laine, M. et al. Atomic proximity between S4 segment and pore domain in Shaker potassium channels. Neuron 39, 467–481 (2003). A rigorous demonstration of disulphide and metal bridge formation between residues in the N terminus of S4 and the C terminus of S5.
Laine, M., Papazian, D. M. & Roux, B. Critical assessment of a proposed model of Shaker. FEBS Lett. 564, 257–263 (2004).
Gandhi, C. S., Clark, E., Loots, E., Pralle, A. & Isacoff, E. Y. The orientation and molecular movement of a K+ channel voltage-sensing domain. Neuron 40, 515–525 (2003).
Broomand, A., Mannikko, R., Larsson, H. P. & Elinder, F. Molecular movement of the voltage sensor in a K+ channel. J. Gen. Physiol. 122, 741–748 (2003).
Ahern, C. A. & Horn, R. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 27, 303–307 (2004).
Cohen, B. E., Grabe, M. & Jan, L. Y. Answers and questions from the KvAP structures. Neuron 39, 395–400 (2003).
Ruta, V., Jiang, Y., Lee, A., Chen, J. & MacKinnon, R. Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature 422, 180–185 (2003).
Miller, C. The charybdotoxin family of K+ channel-blocking peptides. Neuron 15, 5–10 (1995).
Swartz, K. J. & MacKinnon, R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron 18, 665–673 (1997).
Swartz, K. J. & MacKinnon, R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18, 675–682 (1997).
Hong, K. H. & Miller, C. The lipid-protein interface of a Shaker K+ channel. J. Gen. Physiol. 115, 51–58 (2000).
Monks, S. A., Needleman, D. J. & Miller, C. Helical structure and packing orientation of the S2 segment in the Shaker K+ channel. J. Gen. Physiol. 113, 415–423 (1999).
Tu, L., Wang, J., Helm, A., Skach, W. R. & Deutsch, C. Transmembrane biogenesis of Kv1.3. Biochemistry 39, 824–836 (2000).
Cornette, J. L. et al. Hydrophobicity scales and computational techniques for detecting amphipathic structures in proteins. J. Mol. Biol. 195, 659–685 (1987).
Rees, D. C., DeAntonio, L. & Eisenberg, D. Hydrophobic organization of membrane proteins. Science 245, 510–513 (1989).
Li, J., Shi, L. & Karlin, A. A photochemical approach to the lipid accessibility of engineered cysteinyl residues. Proc. Natl Acad. Sci. USA 100, 886–891 (2003).
Karlin, A. & Akabas, M. H. Substituted-cysteine accessibility method. Methods Enzymol. 293, 123–145 (1998).
Jiang, Y., Ruta, V., Chen, J., Lee, A. & MacKinnon, R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423, 42–48 (2003). This study probes the movement of the voltage-sensor paddle motif in KvAP by biotinylating specific sites and assaying for reaction with avidin, added to either external or internal solutions. The authors propose a new paddle model for voltage-sensing in which the paddle motif undergoes a relatively large membrane translocating movement during gating.
Blaustein, R. O. Kinetics of tethering quaternary ammonium compounds to K+ channels. J. Gen. Physiol. 120, 203–216 (2002).
Carter, A., Ketty, V. & Blaustein, R. O. State-dependent reactivity of cysteines substituted into Shaker's gating module. Biophys. J. 86, 192 (2004).
Lee, S. Y. & MacKinnon, R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430, 232–235 (2004).
Takahashi, H. et al. Solution structure of hanatoxin1, a gating modifier of voltage-dependent K+ channels: common surface features of gating modifier toxins. J. Mol. Biol. 297, 771–780 (2000).
Wang, J. M. et al. Molecular surface of tarantula toxins interacting with voltage sensors in Kv channels. J. Gen. Physiol. 123, 455–467 (2004).
Neale, E. J., Elliott, D. J., Hunter, M. & Sivaprasadarao, A. Evidence for intersubunit interactions between S4 and S5 transmembrane segments of the Shaker potassium channel. J. Biol. Chem. 278, 29079–29085 (2003).
Jiang, Q. X., Wang, D. N. & MacKinnon, R. Electron microscopic analysis of KvAP voltage-dependent K+ channels in an open conformation. Nature 430, 806–810 (2004).
Senzel, L., Huynh, P. D., Jakes, K. S., Collier, R. J. & Finkelstein, A. The diphtheria toxin channel-forming T domain translocates its own NH2-terminal region across planar bilayers. J. Gen. Physiol. 112, 317–324 (1998).
Slatin, S. L., Qiu, X. Q., Jakes, K. S. & Finkelstein, A. Identification of a translocated protein segment in a voltage-dependent channel. Nature 371, 158–161 (1994).
Oh, K. J., Senzel, L., Collier, R. J. & Finkelstein, A. Translocation of the catalytic domain of diphtheria toxin across planar phospholipid bilayers by its own T domain. Proc. Natl Acad. Sci. USA 96, 8467–8470 (1999).
Bass, R. B. et al. The structures of BtuCD and MscS and their implications for transporter and channel function. FEBS Lett. 555, 111–115 (2003).
Bass, R. B., Strop, P., Barclay, M. & Rees, D. C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 (2002).
Fersht, A. Structure and Mechanism in Protein Science: a Guide to Enzyme Catalysis and Protein Folding 336–337 (W. H. Freeman, New York, 1999).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy (Kluwer Academic/Plenum, New York, 1999).
Blunck, R., Starace, D. M., Correa, A. M. & Bezanilla, F. Detecting rearrangements of Shaker and NaChBac in real-time with fluorescence spectroscopy in patch-clamped mammalian cells. Biophys. J. 86, 3966–3980 (2004).
Durell, S. R., Hao, Y. & Guy, H. R. Structural models of the transmembrane region of voltage-gated and other K+ channels in open, closed, and inactivated conformations. J. Struct. Biol. 121, 263–284 (1998).
Blaustein, R. O., Cole, P. A., Williams, C. & Miller, C. Tethered blockers as molecular 'tape measures' for a voltage-gated K+ channel. Nature Struct. Biol. 7, 309–311 (2000).
Cuello, L., Cortes, D. M. & Perozo, E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 306, 491–495 (2004). A timely EPR study investigating the mobility and environmental exposure (water vs lipid) of spin labels attached to specific positions throughout S1–S4.
Gutberlet, T. & Katsaras, J. Lipid Bilayers: Structure and Interactions (Springer, Berlin; New York, 2001).
Perozo, E., MacKinnon, R., Bezanilla, F. & Stefani, E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron 11, 353–358 (1993).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982).
Acknowledgements
I thank M. Holmgren, Z. Lu, J. Mindell, E. Perozo, S. Silberberg and the members of the Swartz laboratory for helpful discussions.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Encyclopedia of Life Sciences
Sodium, calcium and potassium channels
Glossary
- MEMBRANE CONDUCTANCE
-
The movement of charged ions across biological membranes gives rise to an electrical current. Conductance is a measure of how readily these currents flow across the membrane.
- HYPERPOLARIZATION/DEPOLARIZATION
-
In most electrically polarized cell membranes the intracellular side is more negatively charged relative to the extracellular side, and the voltage across the membrane is said to be negative. Depolarization signifies a change in membrane voltage whereby the inside becomes more positive; hyperpolarization a change whereby the inside becomes more negative.
- X-RAY STRUCTURE
-
In X-ray crystallography of proteins, a crystallized protein is bombarded with X-rays and the diffraction pattern is used to develop a three dimensional model of the protein's atomic structure. This model is often termed the X-ray structure of the protein.
- ELECTRON PARAMAGNETIC RESONANCE
-
(EPR). A spectroscopic technique based on the magnetic moment of an unpaired free electron. Although proteins typically have little EPR signal, spin-labels with unpaired electrons can be attached to the protein and EPR used to provide information about the mobility and solvent accessibility of the spin-label. EPR can also be used to obtain distances between spin labels and another paramagnetic atom.
- METAL BRIDGE
-
A bridge formed by the coordination of a metal ion by two or more amino acid side chains. The most common bridges use the metals Cd2+ and Zn2+, and involve coordination by cysteine and histidine residues.
- FLUORESCENCE
-
The process by which light is emitted from a substance, typically an aromatic molecule, when an electron in an excited singlet state returns to the ground state. Fluorescence emissions tend to be sensitive to the environment surrounding the fluorophore.
- FAB FRAGMENTS
-
Cleavage of an immunoglobulin with papain releases two Fab fragments, each capable of recognizing antigen, and a single Fc fragment. Fab fragments that recognize motifs in membrane proteins facilitate the production of well-ordered crystals because they can provide additional protein contacts within the crystal and can preclude the protein–detergent micelle from participating in crystal contacts.
- SECONDARY STRUCTURE
-
The local conformation of the polypeptide backbone of a protein can adopt two types of secondary structure that are stabilized by regular hydrogen-bonding interactions between backbone carbonyl and amide groups. The most common secondary structure is the right-handed α-helix, which contains 3.6 residues per turn. The other secondary structure is the β-sheet, which is stabilized by hydrogen bonds between carbonyl and amide groups on two separate β-strands.
- TERTIARY STRUCTURE
-
Tertiary structure refers to the higher order arrangement of secondary protein structures, including loops and linkers, to form the three dimensional structure of the protein.
- AVIDIN
-
A 57 kDa tetrameric protein from egg white that binds the vitamin biotin with extremely high affinity (Kd ∼10−15 M). Biotin derivatives are available that can be tethered to proteins using various reactions, including malemide chemistry for attachment to cysteine residues.
- FLUORESCENCE RESONANCE ENERGY TRANSFER
-
(FRET). The process by which an excited fluorophore (the donor) transfers energy to another molecule (the acceptor) when the emissions spectrum of the donor overlaps the absorption spectrum of the acceptor. The distance over which energy transfer is 50% efficient, the Förster distance, is typically in the range of 20–60 Å, making FRET useful as a spectroscopic ruler for distance measurements in proteins.
Rights and permissions
About this article
Cite this article
Swartz, K. Towards a structural view of gating in potassium channels. Nat Rev Neurosci 5, 905–916 (2004). https://doi.org/10.1038/nrn1559
Issue Date:
DOI: https://doi.org/10.1038/nrn1559
This article is cited by
-
New Structural insights into Kir channel gating from molecular simulations, HDX-MS and functional studies
Scientific Reports (2020)
-
Allosteric coupling between proximal C-terminus and selectivity filter is facilitated by the movement of transmembrane segment 4 in TREK-2 channel
Scientific Reports (2016)
-
CHEXVIS: a tool for molecular channel extraction and visualization
BMC Bioinformatics (2015)
-
Conformational Dynamics of Shaker-Type Kv1.1 Ion Channel in Open, Closed, and Two Mutated States
The Journal of Membrane Biology (2015)
-
Water wettability in nanoconfined environment
Science China Physics, Mechanics & Astronomy (2014)