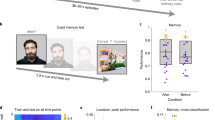
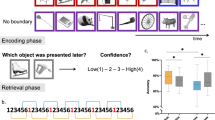
Abstract
Remembering information from continuous past episodes is a complex task1. On the one hand, we must be able to recall events in a highly accurate way, often including exact timings. On the other hand, we can ignore irrelevant details and skip to events of interest. Here, we track continuous episodes consisting of different subevents as they are recalled from memory. In behavioural and magnetoencephalography data, we show that memory replay is temporally compressed and proceeds in a forward direction. Neural replay is characterized by the reinstatement of temporal patterns from encoding2,3. These fragments of activity reappear on a compressed timescale. Herein, the replay of subevents takes longer than the transition from one subevent to another. This identifies episodic memory replay as a dynamic process in which participants replay fragments of fine-grained temporal patterns and are able to skip flexibly across subevents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Group statistical data relating to this project are deposited in a public repository (https://doi.org/10.25500/eData.bham.00000254). The individual data that support the findings of this study are available from the corresponding author upon request.
References
Tulving, E. What is episodic memory? Curr. Dir. Psychol. Sci. 2, 67–70 (1993).
Michelmann, S., Bowman, H. & Hanslmayr, S. The temporal signature of memories: identification of a general mechanism for dynamic memory replay in humans. PLoS Biol. 14, e1002528 (2016).
Michelmann, S., Bowman, H. & Hanslmayr, S. Replay of stimulus-specific temporal patterns during associative memory formation. J. Cogn. Neurosci. 30, 1577–1589 (2018).
Arnold, A. E. G. F., Iaria, G. & Ekstrom, A. D. Mental simulation of routes during navigation involves adaptive temporal compression. Cognition 157, 14–23 (2016).
Bonasia, K., Blommesteyn, J. & Moscovitch, M. Memory and navigation: compression of space varies with route length and turns. Hippocampus 26, 9–12 (2016).
Foster, D. J. & Wilson, M. A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683 (2006).
Carr, M. F., Jadhav, S. P. & Frank, L. M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 14, 147–153 (2011).
Yaffe, R. B., Shaikhouni, A., Arai, J., Inati, S. K. & Zaghloul, K. A. Cued memory retrieval exhibits reinstatement of high gamma power on a faster timescale in the left temporal lobe and prefrontal cortex. J. Neurosci. 37, 4472–4480 (2017).
Staudigl, T., Vollmar, C., Noachtar, S. & Hanslmayr, S. Temporal-pattern similarity analysis reveals the beneficial and detrimental effects of context reinstatement on human memory. J. Neurosci. 35, 5373–5384 (2015).
Zhang, H. et al. Gamma power reductions accompany stimulus-specific representations of dynamic events. Curr. Biol. 25, 635–640 (2015).
Wimber, M., Maaß, A., Staudigl, T., Richardson-Klavehn, A. & Hanslmayr, S. Rapid memory reactivation revealed by oscillatory entrainment. Curr. Biol. 22, 1482–1486 (2012).
Chen, J. et al. Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 20, 115–125 (2017).
Kriegeskorte, N. Representational similarity analysis—connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 1–28 (2008).
Staresina, B. P. et al. Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. eLife 5, e17397 (2016).
Yaffe, R. B. et al. Reinstatement of distributed cortical oscillations occurs with precise spatiotemporal dynamics during successful memory retrieval. Proc. Natl Acad. Sci. USA 111, 18727–18732 (2014).
Kurth-Nelson, Z., Barnes, G., Sejdinovic, D., Dolan, R. & Dayan, P. Temporal structure in associative retrieval. eLife 4, e04919 (2015).
Ng, B. S. W., Logothetis, N. K. & Kayser, C. EEG phase patterns reflect the selectivity of neural firing. Cereb. Cortex 23, 389–398 (2013).
Schyns, P. G., Thut, G. & Gross, J. Cracking the code of oscillatory activity. PLoS Biol. 9, e1001064 (2011).
Lachaux, J.-P. et al. Studying single-trials of phase-synchronous activity in the brain. Int. J. Bifurc. Chaos 10, 2429–2439 (2000).
Mormann, F., Lehnertz, K., David, P. & Elger, E. C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D 144, 358–369 (2000).
Ji, D. & Wilson, M. A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007).
Albers, A. M., Kok, P., Toni, I., Dijkerman, H. C. & de Lange, F. P. Shared representations for working memory and mental imagery in early visual cortex. Curr. Biol. 23, 1427–1431 (2013).
Ekman, M., Kok, P. & de Lange, F. P. Time-compressed preplay of anticipated events in human primary visual cortex. Nat. Commun. 8, 15276 (2017).
Bosch, S. E., Jehee, J. F. M., Fernandez, G. & Doeller, C. F. Reinstatement of associative memories in early visual cortex is signaled by the hippocampus. J. Neurosci. 34, 7493–7500 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Radvansky, G. A. & Zacks, J. M. Event boundaries in memory and cognition. Curr. Opin. Behav. Sci. 17, 133–140 (2017).
Sols, I., DuBrow, S., Davachi, L. & Fuentemilla, L. Event boundaries trigger rapid memory reinstatement of the prior events to promote their representation in long-term memory. Curr. Biol. 27, 3499–3504.e4 (2017).
Davachi, L. & DuBrow, S. How the hippocampus preserves order: the role of prediction and context. Trends Cogn. Sci. 19, 92–99 (2015).
Buzsáki, G. & Tingley, D. Space and time: the hippocampus as a sequence generator. Trends Cogn. Sci. 22, 853–869 (2018).
Johnson, A. & Redish, A. D. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J. Neurosci. 27, 12176–12189 (2007).
Jafarpour, A., Fuentemilla, L., Horner, A. J., Penny, W. & Duzel, E. Replay of very early encoding representations during recollection. J. Neurosci. 34, 242–248 (2014).
Coltheart, M. The MRC psycholinguistic database. Q. J. Exp. Psychol. A 33, 497–505 (1981).
Brysbaert, M. & New, B. Moving beyond Kučera and Francis: a critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav. Res. Methods 41, 977–990 (2009).
Brainard, D. H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Ratcliff, R. Group reaction time distributions and an analysis of distribution statistics. Psychol. Bull. 86, 446–461 (1979).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 1–9 (2011).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Tal, I. & Abeles, M. Cleaning MEG artifacts using external cues. J. Neurosci. Methods 217, 31–38 (2013).
Stolk, A., Todorovic, A., Schoffelen, J. M. & Oostenveld, R. Online and offline tools for head movement compensation in MEG. Neuroimage 68, 39–48 (2013).
Long, N. M., Burke, J. F. & Kahana, M. J. Subsequent memory effect in intracranial and scalp EEG. NeuroImage 84, 488–494 (2014).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 51, 273–289 (2002).
Tallon-Baudry, C., Bertrand, O., Delpuech, C. & Pernier, J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 16, 4240–4249 (1996).
Busch, N. A., Dubois, J. & VanRullen, R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 29, 7869–7876 (2009).
Hentschke, H. & Stüttgen, M. C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 34, 1887–1894 (2011).
Rouder, J. N., Speckman, P.L., Sun, D., Morey, R. D. & Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 16, 225–237 (2009).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190 (2007).
Kriegeskorte, N., Simmons, W. K., Bellgowan, P. S. & Baker, C. I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 12, 535–540 (2009).
Acknowledgements
The authors thank the SPMIC—specifically G. O’Neill, B. A. E. Hunt and L. Gascoyne—for help with data collection. This work was supported by the ERC Grant Code4Memory (647954) awarded to S.H., who is further supported by the Wolfson Society and Royal Society. B.P.S. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (107672/Z/15/Z). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.M., B.P.S. and S.H. conceived and designed the experiments. S.M. performed the experiments. S.M. analysed the data under the supervision of S.H. S.M., S.H. and H.B. contributed reagents, materials and analysis tools. S.M. and S.H. wrote the paper, with comments and edits by B.P.S. and H.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes, Supplementary Methods, Supplementary Figures 1–7
Rights and permissions
About this article
Cite this article
Michelmann, S., Staresina, B.P., Bowman, H. et al. Speed of time-compressed forward replay flexibly changes in human episodic memory. Nat Hum Behav 3, 143–154 (2019). https://doi.org/10.1038/s41562-018-0491-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0491-4
This article is cited by
-
Neural signatures associated with temporal compression in the verbal retelling of past events
Communications Biology (2022)
-
Dynamics of fMRI patterns reflect sub-second activation sequences and reveal replay in human visual cortex
Nature Communications (2021)
-
Moment-by-moment tracking of naturalistic learning and its underlying hippocampo-cortical interactions
Nature Communications (2021)
-
Flexible modulation of sequence generation in the entorhinal–hippocampal system
Nature Neuroscience (2021)
-
Time-frequency feature extraction for classification of episodic memory
EURASIP Journal on Advances in Signal Processing (2020)