Abstract
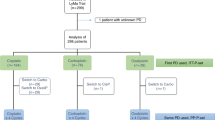
The purpose of this study was to develop a high-dose chemotherapy (HDC) and peripheral blood stem cell (PBSC) regimen for treatment of patients with ovarian carcinoma that could be administered in an outpatient setting. Fourteen patients with advanced ovarian (n = 9) or breast (n = 5) carcinoma, who had failed conventional chemotherapy, were entered into a dose-escalation trial to determine the maximum tolerated dose (MTD) of carboplatin that could be administered with fixed doses of melphalan (160 mg/m2) and mitoxantrone (50 mg/m2). Twenty-five additional patients were included in a phase II trial at the MTD. Two of two patients had grade 4 severe regimen-related toxicities (RRT), one fatal, at a dose level of 1600 mg/m2. Two of 29 patients (6.9%) treated at the MTD (carboplatin, 1400 mg/m2) died of RRT. All three patients who died of toxicity had a calculated AUC for carboplatin >30 mg/ml/min. Thirty-one patients with ovarian cancer who had failed chemotherapy were treated, 24 at the MTD. Fourteen of 20 patients (70%) with ovarian carcinoma with evaluable disease achieved a CR and seven (35%) are alive disease-free a median of 20 months (range, 7–26). Five of seven patients with ovarian cancer who had failed chemotherapy but were rendered clinically disease-free following surgery survive without progression a median of 13 months (range, 9–19). Eight of 16 (50%) platinum-resistant and 4/12 (33%) platinum-sensitive patients with ovarian cancer survive disease-free.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weaver, C., Greco, F., Hainsworth, J. et al. A phase I–II study of high-dose melphalan, mitoxantrone and carboplatin with peripheral blood stem cell support in patients with advanced ovarian or breast carcinoma. Bone Marrow Transplant 20, 847–853 (1997). https://doi.org/10.1038/sj.bmt.1700976
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1700976