Abstract
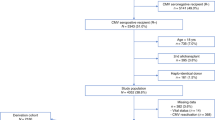
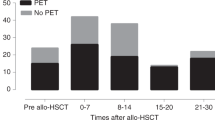
We prospectively evaluated a risk-adapted pre-emptive treatment with ganciclovir for CMV diseases in patients undergoing allogeneic bone marrow transplantation (BMT). High-level CMV antigenemia (10 or more positive cells on two slides) or CMV antigenemia at any level in patients with grade II–IV acute graft-versus-host disease (aGVHD) were chosen as risk factors. We also retrospectively evaluated virus reactivation in plasma using quantitative real-time polymerase chain reaction (PCR). Fifty patients were evaluable. None of the 27 patients with or without grade I aGVHD developed high-level CMV antigenemia or CMV disease. Among the 23 patients with grade II–IV aGVHD, 12 patients (52%) developed CMV antigenemia and were treated pre-emptively, of whom two developed CMV gastroenteritis or retinitis in spite of therapy. Six of the remaining 11 patients developed CMV gastroenteritis before CMV antigenemia was detectable. All of the eight patients with CMV diseases were successfully treated with ganciclovir and no deaths directly related to CMV disease occurred. In four of the seven evaluable patients with CMV gastroenteritis, real-time PCR was able to detect virus reactivation earlier than CMV antigenemia. Although our risk-adapted pre-emptive therapy effectively reduced CMV-related mortality, further refinements of this approach, particularly in the prevention of CMV gastroenteritis, may be achieved by incorporating real-time PCR. Bone Marrow Transplantation (2000) 25, 765–769.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Myers JD, Flournoy N, Thomas ED . Risk factors for cytomegalovirus infection after human marrow transplantation J Infect Dis 1986 153: 478–488
Reed EC, Bowden RA, Dandliker PS et al. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants Ann Intern Med 1988 15: 783–788
Goodrich JM, Bowden RA, Fisher L et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant Ann Intern Med 1993 118: 173–178
Winston DJ, Bartoni K, Du Mond C et al. Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients Ann Intern Med 1993 118: 179–184
Goodrich JM, Mori M, Gleaves CA et al. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation New Engl J Med 1991 325: 1601–1607
Schmidt GM, Horak D, Niland JC et al. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants New Engl J Med 1991 324: 1005–1011
Boeckh M, Gooley TA, Myerson D et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study Blood 1996 88: 4063–4071
Manteiga R, Martino R, Sureda A et al. Cytomegalovirus pp65 antigenemia-guided pre-emptive treatment with ganciclovir after allogeneic stem cell transplantation: a single-center experience Bone Marrow Transplant 1998 22: 899–904
Heid CA, Stevens J, Livak KJ et al. Real time quantitative PCR Genome Res 1996 6: 986–994
Przepiorka D, Weisdorf D, Martin P et al. Consensus conference on acute GVHD grading Bone Marrow Transplant 1995 15: 825–828
Kurihara T, Hayashi J, Matusoka T et al. HCMV pp65 antigenemia assay using indirect alkaline phosphatase staining method Biomed Res 1995 16: 125–129
Einsele H, Ehninger G, Hebart H et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side-effects of antiviral therapy after bone marrow transplantation Blood 1995 86: 2815–2820
Ljungman P, Lore K, Aschan J et al. Use of a semi-quantitative PCR for cytomegalovirus DNA as a basis for pre-emptive antiviral therapy in allogeneic bone marrow transplant patients Bone Marrow Transplant 1996 17: 583–587
Kanda Y, Chiba S, Suzuki T et al. Time course analysis of semi-quantitative PCR and antigenemia assay for prevention of cytomegalovirus disease after bone marrow transplantation Br J Haematol 1998 100: 222–225
Gor D, Sabin C, Prentice HG et al. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease Bone Marrow Transplant 1998 21: 597–605
Li C-R, Greenberg PD, Gilvert MJ et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplantation: correlation with CMV disease and effect of ganciclovir prophylaxis Blood 1994 83: 1971–1979
Acknowledgements
The authors thank the attending doctors, dedicated fellows, and nurses in Keio BMT program for providing excellent patient care. The authors also thank Dr John Wingard for his kind suggestions in preparing this manuscript. This work was supported in part by grants from the Japan Society for the Promotion of Science (JSPS-RFTF 97L00701) and the Ministry of Education, Science and Culture of Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mori, T., Okamoto, S., Matsuoka, S. et al. Risk-adapted pre-emptive therapy for cytomegalovirus disease in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant 25, 765–769 (2000). https://doi.org/10.1038/sj.bmt.1702227
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702227
Keywords
This article is cited by
-
Cytomegalovirus retinitis after allogeneic hematopoietic stem cell transplantation under cytomegalovirus antigenemia-guided active screening
Bone Marrow Transplantation (2021)
-
Impact of cytomegalovirus gastrointestinal disease on the clinical outcomes in patients with gastrointestinal graft-versus-host disease in the era of preemptive therapy
Annals of Hematology (2013)
-
Cytomegalovirus infection causes morbidity and mortality in patients with autoimmune diseases, particularly systemic lupus: in a Chinese population in Taiwan
Rheumatology International (2012)
-
Initial low-dose valganciclovir as a preemptive therapy is effective for cytomegalovirus infection in allogeneic hematopoietic stem cell transplant recipients
International Journal of Hematology (2012)
-
Pyrosequencing allows the detection of emergent ganciclovir resistance mutations after HCMV infection
Medical Microbiology and Immunology (2011)