Abstract
Previous studies demonstrated that Gsα migrates from a Triton X-100 (TTX-100) insoluble membrane domain to a TTX-100 soluble membrane domain in response to chronic treatment with the antidepressants desipramine and fluoxetine. Antidepressant treatment also causes a Gsα redistribution in cells as seen by confocal microscopy. The current studies have focused on examining the possibility that the association between Gsα and the plasma membrane and/or cytoskeleton is altered in response to antidepressant treatment, and that this is relevant to both Gsα redistribution and the increased coupling between Gsα and adenylyl cyclase seen after chronic antidepressant treatment. Chronic treatment of C6 cells with two fuctionally and structurally distinct antidepressants, desipramine and fluoxetine, decreased the Gsα content of TTX-100 insoluble membrane domains by as much as 60%, while the inactive fluoxetine analog LY368514 had no effect. Disruption of these membrane domains with the cholesterol chelator methyl-β-cyclodextrin altered the localization of many proteins involved in the cAMP signaling cascade, but only Gsα localization was altered by antidepressant treatment. In addition, microtubule disruption with colchicine elicited the movement of Gsα out of detergent-resistant membrane domains in a manner identical to that seen with antidepressant treatment. The data presented here further substantiate the role of Gsα as a major player in antidepressant-induced modification of neuronal signaling and also raise the possibility that an interaction between Gsα and the cytoskeleton is involved in this process.
Similar content being viewed by others
INTRODUCTION
For over half a century, electroconvulsive shock (ECS) and various classes of antidepressant drugs have been used for the treatment of depression and other psychiatric disorders. The possibility that these diverse agents converge on a single postsynaptic target has been an area of great research interest. In 1983, we first reported that long-term administration of various antidepressants enhanced guanylyl-5′-imidodiphoshate (Gpp(NH)p)- and fluoride-stimulated adenylyl cyclase activity in rat cortex and hypothalamus membranes (Menkes et al, 1983). This suggested that the stimulatory α-subunit of the G protein, Gs, was at least an indirect target of antidepressant action, and that antidepressant treatment facilitated the activation of adenylyl cyclase by Gs. These initial findings have been substantiated by later in vivo and in vitro studies (Chen and Rasenick, 1995a; De Montis et al, 1990; Kamada et al, 1999; Ozawa and Rasenick, 1989, 1991; Perez et al, 1991, 1989). Consistent with these findings, it has been reported that chronic antidepressant treatment increases the expression and activity of cAMP response element binding protein (CREB) in the rat brain (Duman et al, 1997; Nibuya et al, 1996; Takahashi et al, 1999; Thome et al, 2000).
G protein signaling complexes at the plasma membrane have been identified as associated with specific components of the membrane and cytoskeleton (Huang et al, 1997). These domains, which contain receptors, G proteins and effector enzymes, and other proteins, are likely to be constrained from lateral mobility within the plasma membrane (Neubig, 1994) in part by cytoskeletal structures that form ‘corrals’ on the inner membrane face (Kuo and Sheetz, 1993). Certain G proteins have also been shown to bind to tubulin (Wang et al, 1990) and microtubules (Sarma et al, 2003). This appears to both modulate cytoskeletal dynamics and cell signaling.
The localization of G proteins to specific detergent insoluble membrane domains such as caveolae (Li et al, 1995) and rafts has generated interest as to how these cholesterol and sphingolipid-rich detergent-resistant membrane domains modulate G protein targeting and function (Bayewitch et al, 2000; Brown and London, 2000; Moffett et al, 2000; Ostrom and Insel, 2004). Interestingly, the different G protein α subunits are localized in distinct plasma membrane domains (Oh and Schnitzer, 2001). While both Gsα and Giα can move in and out of caveolae, they are predominantly found in lipid rafts complexed with Gβγ. On the other hand, Gqα couples to caveolin and is found predominantly in caveolae without Gβγ. Current studies in this laboratory have found that Gsα raft localization is increased after isoproteronol stimulation (Allen et al, 2004, submitted).
Recent studies have demonstrated that Gsα from C6 rat glioma cells migrates from a caveolae and raft-enriched Triton X-100 (TTX-100) insoluble membrane domain (from here on referred to as a Triton Insoluble Floating Fraction (TIFF) due to the ability to float on sucrose gradients) to a TTX-100 soluble membrane domain in response to chronic antidepressant treatment (Toki et al, 1999). The TTX-100 detergent extractability of Gsα from C6 cell or rat brain membranes was increased upon treatment with amitriptyline, desipramine, and fluoxetine. Gsα, which is normally enriched in TIFFs (that include caveolae), was removed from these domains by 50% in antidepressant-treated C6 cells. There was no shift in the membrane localization of Giα to a more TTX-100 soluble membrane domain after antidepressant treatment, suggesting that the antidepressant effect on G protein membrane localization is Gsα specific (Toki et al, 1999). These data are reinforced by the findings of Bayewitch et al (2000) who showed that chronic exposure to agonists of Giα- coupled receptors leads to a decrease in the cholate solubility of these G protein subunits and a ‘superactivation’ of adenylyl cyclase. Further studies involving the visualization of Gsα demonstrated that Gsα is localized to the plasma membrane as well as the cytosol in both desipramine-treated and nontreated control cells (Donati et al, 2001). In nontreated cells, Gsα is distributed throughout the entire length of the cell processes with an enrichment at the distal end. Antidepressant-treated cells show reduced localization of Gsα in cellular processes (especially the distal ends), while Gsα is increased in the perinuclear region of the cells (Donati et al, 2001). Taken together, these studies suggest that the lipid environment and cytoskeletal association of the G protein may play an important role in its localization and function, and that chronic antidepressant treatment alters the membrane localization of Gsα, resulting in augmented coupling to adenylyl cyclase.
The aim of this study is to further investigate the role of antidepressants on the membrane and cytoskeletal localization of Gsα. We examined whether antidepressants effect Gsα membrane localization via disruption of the raft domains or disruption of the microtubule cytoskeleton. In addition to the chronic antidepressant treatment regimen, these cells were treated with a fluoxetine analog that differs only by the position of the CF3 group to determine if structurally similar compounds could exert a similar effect on Gsα localization. Furthermore, the cells were acutely treated with the cholesterol chelator methyl-β-cyclodextrin or the microtubule-disrupting agent colchicine. The results of this study directly reveal that the localization of Gsα is a prime target for antidepressant action. This study also lends support to the hypothesis that the microtubule cytoskeleton and its regulation of G protein signaling is involved in the sequellae of events initiated by antidepressant treatment that results in altered Gsα signaling.
METHODS
Cell Culture and Treatment Paradigms
C6 cells (between passages 30 and 50) were grown in 150 cm2 flasks and allowed to attach overnight in Dulbecco's modified Eagle's medium, 4. 5 g of glucose/l, 10% newborn calf serum (Hyclone), 100 μg/ml penicillin and streptomycin at 37°C in humidified 10% CO2 atmosphere. As reported previously, desipramine treatment regimens of 3 μM for 5 days and 10 μM for 3 days yielded similar biochemical results (Chen and Rasenick, 1995b). Therefore, the latter treatment paradigm was used in these experiments because it was easier to maintain the cell cultures for 3 days. The cells were treated with 10 μM of either desipramine hydrochloride (Sigma), fluoxetine, or LY368514 (both gifts from Eli Lilly and Co., structure in Figure 1a) for 3 days or 10 mM methyl-β cyclodextrin (Sigma) for 30 min. The culture media and drug were changed daily. None of the treatments altered cell growth (as determined by the confluency of the cell monolayer and total protein estimation). In a separate experiment, C6 cells were treated with desipramine as above while another group of cells was treated with colchicine (2 and 5 μM) and β-lumicolchicine (5 μM) (both from Sigma) for 2 h prior to harvesting.
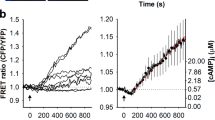
Antidepressant treatment of C6 cells causes a shift in the localization of Gsα from a TTX-100 insoluble lipid-raft-rich domain to a more TTX-100 soluble domain. (a) Chemical backbone of fluoxetine with the CF3 group in the para position (4) and LY368514 with the CF3 group in the ortho position (2). (b) Cells were treated chronically with desipramine (DMI), fluoxetine (FLU), or the inactive fluoxetine analog, LY368514 (LY) (3 days, 10 μM). The detergent insoluble membranes were floated on sucrose density gradients and analyzed by SDS-PAGE and immunoblot for Gsα content. Representative immunoblot from three experiments. (c) Percent change in Gsα in the TTX-100 insoluble lipid-raft-rich fraction. Note that acute treatment has no effect on the redistribution of Gsα. Autoradiographs were compared by densitometry from three separate experiments and the results were plotted using Prism Graphpad 4.0. Data were analyzed by one-way ANOVA followed by Newman–Keuls multiple comparison post-test. Mean±SEM (**p<0.01, ***p<0.001).
Cell Fractionation by Sucrose Density Gradient Sedimentation
Following treatment, cells were washed, scraped and sedimented and the pellets were used to prepare TIFFs by the procedure of Li et al (1995) with minor modifications. Briefly, C6 cells were harvested into 0.75 ml of HEPES buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM DTT, protease inhibitors) containing 1% TTX-100. Homogenization was carried out with 10 strokes of a Potter–Elvehjem homogenizer. The homogenate was adjusted to 40% sucrose by the addition of an equal volume of 80% sucrose prepared in HEPES buffer and placed at the bottom of an ultracentrifuge tube. A step gradient containing 30, 15, and 5% sucrose was formed above the homogenate and centrifuged at 50 000 rpm for 20 h in an SW65 rotor (≈240 000 g). The opaque bands confined between the 15 and 30% sucrose layers were harvested, diluted three-fold with HEPES buffer and pelleted in a microcentrifuge at 16 000 g. The pellet was resuspended in HEPES buffer and subjected to further biochemical analysis.
SDS-PAGE and Immunoblotting
TIFFs were analyzed by SDS-PAGE followed by Western blotting onto PVDF membranes (Millipore). Proteins were detected with antibodies to protein kinase A (PKA) catalytic and regulatory subunits, adenylyl cyclase type II and V/VI (Santa Cruz), tubulin (Sigma), actin C4 clone (gift from Dr J Lessard), and Gsα (Dupont/NEN) followed by secondary antibody (HRP-linked anti-rabbit or mouse IgG F(ab′)2) (Jackson). Immunoreactivity was detected with an enhanced chemiluminescence (ECL) Western blot detection system (Amersham and Pierce) in accord with the manufacturer's instructions. Autoradiographs were analyzed by densitometry using ImageQuant software (Molecular Dynamics).
Cholesterol Analysis
C6 membranes were prepared as described (Rasenick and Kaplan, 1986) and stored under liquid N2 until use. C6 whole cell lysates (hypotonic lysis on ice) and 100 000 g crude membrane pellets of the lysates (both in 15 mM HEPES, pH 7.4) were submitted to the Clinical Pathology Department at UIC for cholesterol analysis. Samples were analyzed on the SYNCHRON CX System using the Cholesterol Reagent kit in conjunction with the SYNCHRON Systems CX MULTI Calibrator (Beckman Coulter). The reaction results in the hydrolysis of cholesterol esters into free cholesterol and the oxidation of free cholesterol producing hydrogen peroxide. The result is quantified by a peroxidase-generated color reaction.
Statistical Methods
Data were analyzed for statistical significance using a one-way ANOVA followed by Student–Newman–Keuls multiple comparison test using Prism 4.0, software package for statistical data analysis (Graph Pad Software Inc.). Additionally, a one sample T-test using a hypothetical mean of 100% for the control samples was performed (Table 1). Means are ±SEM and differences for all experiments were considered significant at p<0.05.
RESULTS
Antidepressants Effect Gsα Membrane Domain Localization
Previous results demonstrated that the cellular localization of Gsα is altered in response to chronic, but not acute antidepressant treatment (Donati et al, 2001; Toki et al, 1999). These studies showed that Gsα localization to TIFFs was decreased after chronic antidepressant, but not chronic antipsychotic drug (chlorpromazine) nor nonantidepressant uptake inhibitor (amphetamine) treatment (Toki et al, 1999), and under these same conditions, it moved out of the process tips of C6 glioma cells (Donati et al, 2001). The membrane localization of caveolin-1, which is a membrane protein highly enriched in TIFFs, was not altered by similar antidepressant treatments (Toki et al, 1999). To confirm these results, we used the inactive fluoxetine analog LY368514 under chronic treatment conditions (Figure 1). LY368514 differs from fluoxetine by having a CF3 group in the para vs the ortho position (Figure 1a) and is ineffective at inhibiting 5HT transport (Wong et al, 1995). Figure 1b is a representative immunoblot showing the reduced amount of Gsα in the TIFFs of antidepressant-treated cells compared to drug-free control cells. Notice that LY368514 did not decrease the amount of Gsα in the TIFFs. The results of three such experiments are quantified in Figure 1c where Gsα was reduced to 50 and 35% of control levels by desipramine and fluoxetine, respectively, while LY368514 had no effect on the amount of Gsα in the TIFFs.
Lipid Raft Disruption and Cellular Compartmentalization of Gsα
Cholesterol-depleting compounds, such as cyclodextrin, cause a disruption of the raft/caveolae structure by chelating the cholesterol that gives these domains their rigid structure (Miura et al, 2001; Nichols, 2003). Tricyclic antidepressants have been also shown to alter fluorescence anisotropy, suggesting a possible reordering of membrane lipids (Menkes et al, 1983). Thus, we sought to compare cyclodextrin and desipramine treatment with respect to effects on a number of membrane proteins within lipid raft domains represented by TIFFs. We chose to use only desipramine in the following studies because the effects of desipramine and fluoxetine on Gsα TIFF localization have been shown to be identical (Figure 1 and Toki et al, 1999). C6 glioma cells were treated with cyclodextrin (acute) or desipramine (chronic) as described in the Methods section. For cholesterol analysis, total cell lysates and crude membrane preparations were examined (Figure 2). TTX-100 detergent extracts from the treated C6 cells were subjected to sucrose density ultracentrifugation for TIFF isolation and protein analysis (Figure 3). Methyl-β-cyclodextrin treatment resulted in a significant (40%) decrease in the amount of membrane cholesterol compared to the control sample, while desipramine had no significant effect (Figure 2). Lipid rafts (TIFFs) were examined for proteins involved in the cAMP signaling cascade (adenylyl cyclase types II and V/VI, PKA regulatory and catalytic subunits, Gsα) and cytoskeletal proteins (tubulin and actin). While many of the proteins analyzed (Gsα, ACII, PKAcat, and PKAreg) were significantly removed from TIFFs due to cholesterol depletion of these membrane domains, only Gsα content was significantly changed by chronic desipramine treatment (Figure 3 & Table 1). Thus, even though chronic antidepressant treatment may have an effect on membrane lipids, Gsα appears to be a unique target of that effect.
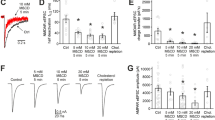
Desipramine treatment does not effect the total or membrane cholesterol content of C6 glioma cells. Total cholesterol levels were measured from C6 cell lysates and membrane cholesterol levels were measured from C6 membrane preparations and corrected for the amount of protein in the preparations. Treatment of cells with cyclodextrin as described in Methods section depleted approximately 50% of the total and membrane cholesterol, while chronic desipramine treatment had no effect on membrane cholesterol. Results from four separate experiments were plotted using Prism Graphpad 4.0. Data were analyzed by one-way ANOVA followed by Newman–Keuls multiple comparison post-test. Mean±SEM (**p<0.01).
Gsα membrane localization is disrupted by antidepressant treatment, while other members of the cAMP signaling cascade are not effected. C6 glioma cells were treated with desipramine and cyclodextrin as described in Methods. In all, 1% Triton X-100 cell lysates were prepared and centrifuged and TIFFs were isolated, washed, and prepared for SDS-PAGE. Western blots were probed for proteins involved in the cAMP signaling cascade (adenylyl cyclase types II and V/VI, PKA regulatory and catalytic subunits, Gsα) and cytoskeletal proteins (tubulin and actin). Blots are a representative of 4–6 separate experiments with the exception of actin, which is 2. C=control, CD=cyclodextrin, DMI=desipramine.
Effects of Colchicine on Gsα Raft Localization
Earlier experiments showed that rat cerebral cortex synaptic membranes treated with microtubule disrupting drugs (colchicine or vinblastine) increased Gsα-stimulated adenylyl cyclase activity due to an apparent release of Gsα from a cytoskeletal ‘tether’, which prevented Gsα from a facile interaction with adenylyl cyclase (Rasenick et al, 1981). We wanted to determine if microtubule disruption in intact C6 cells would have antidepressant-like effects on Gsα raft localization. Colchicine (up to 10 μM) treatment of C6 cells for up to 4 h did not induce toxic effects (Hough et al, 1994). The data in Figure 4 demonstrate that acute treatment (2 h) with colchicine (2 or 5 μM) does in fact have an effect on Gsα raft localization similar to that seen with chronic desipramine treatment, but the two drug treatments are not additive. These treatment regimens resulted in a significant movement of 20–40% of Gsα out of the raft domains. Treatment with the inactive colchicine isomer, β-lumicolchicine, is without effect. The amount of raft localized Gsα compared to control is as follows: desipramine—72.4±6.1%; 2 μM colchicine—80.0±3.6%; 5 μM colchicine—70.0±14%; desipramine/colchicine—63.7±16.4%; and 5 μM β-lumicolchicine—109.0±8.0. Thus, it appears that microtubule disruption had a similar effect on the cellular localization of Gsα as did desipramine treatment. The nonadditive result of these drugs suggests that they may be affecting the membrane distribution of Gsα in a similar manner.
Microtubule disruption and chronic antidepressant treatment have comparable effects on Gsα membrane compartmentalization. C6 cells were treated with desipramine (DMI) as in Figure 1, colchicine (Colc.) (2 or 5 μM for 2 h.), β-lumicolchicine (β-Lum.) (5 μM for 2 h), desipramine followed by 2 μM colchicine (DMI/Colc), or nontreated controls (C). Results from 3–5 separate experiments were plotted on a graph using Prism Graphpad 4.0. Data were analyzed by one-way ANOVA followed by Newman–Keuls multiple comparison post-test. Mean±SEM (*p<0.05, **p<0.01).
DISCUSSION
Over 20 years ago, we first reported that long-term administration of various antidepressants enhanced guanylyl-5′-imidodiphoshate (Gpp(NH)p)- and fluoride-stimulated adenylyl cyclase activity in rat cortex and hypothalamus membranes (Menkes et al, 1983). This suggested that some aspect of Gsα was a target of antidepressant action, and that antidepressant treatment facilitated the activation of adenylyl cyclase by making Gsα more available without altering expression of that protein (Ozawa and Rasenick, 1989; Chen and Rasenick, 1995a). Similar antidepressant-induced increases in Gpp(NH)p-stimulated adenylyl cyclase activity have been observed in vitro using C6 glioma cells (Chen and Rasenick, 1995a). Later studies demonstrated that the membrane localization of Gsα is altered after chronic antidepressant treatment, and that the presence of Gsα in lipid raft/caveolar membrane domains may be important to its function as a mediator of antidepressant action (Donati et al, 2001; Toki et al, 1999).
Postreceptor neuronal cell signaling processes, particularly the downstream effects involving cAMP, have been the focus of many in vivo studies seeking to determine mechanisms of antidepressant action (Duman et al, 1997; Nibuya et al, 1996; Ozawa and Rasenick, 1989; Perez et al, 1991, 1989; Takahashi et al, 1999; Thome et al, 2000). While each of these studies focuses on a different aspect of intracellular signaling, they are all compatible with a sustained increase in cAMP synthesis. Similar effects using in vitro models of antidepressant action have also been observed (Chen and Rasenick, 1995a; Donati et al, 2001; Toki et al, 1999). Toki et al (1999) demonstrated that antidepressant treatment results in an alteration in the detergent extractability of Gsα from the plasma membrane of C6 glioma cells and rat cerebral cortex. Altered detergent solubility of Giα and Gβγ has also been demonstrated after chronic activation of Gi/o-coupled opiate receptors (Bayewitch et al, 2000). This change in detergent solubility corresponds to adenylyl cyclase ‘superactivation’. Most recently, we have demonstrated that Gsα is internalized in the lipid rafts during the process of desensitization. This, at least for Gsα, suggests that rafts are domains where signaling is attenuated (Allen et al, 2004, submitted). (Note that the opposite may hold for other G proteins such as Gqα (Bhatnagar et al, 2004)). The current study confirms that the TTX-100 extractability of Gsα is altered by chronic antidepressant treatment. Additionally, the data presented here further substantiate the role of Gsα as a cornerstone for antidepressant-induced changes in cell signaling and also shed light on the possibility that an interaction between Gsα-tubulin is involved in this signaling process.
The functionally inactive fluoxetine analog LY368514, which differs from fluoxetine by having a CF3 group in the para vs the ortho position and is ineffective at inhibiting 5HT transport (Wong et al, 1995), was unable to decrease the amount of Gsα in C6 TIFFs under chronic treatment conditions (Figure 1). Chronic treatment of these cells with either a tricyclic (desipramine) or a selective serotonin reuptake inhibitor (fluoxetine), two fuctionally and structurally distinct antidepressants, was able to decrease the Gsα content of TIFFs by as much as 60% (Figure 1). We have observed that compounds with structural similarity to antidepressants, but without antidepressant effect, have no effect on Gsα localization, both in this study and in a previous study (Toki et al, 1999). Thus, it appears that movement of Gsα out of TIFFs is induced by chronic antidepressant treatment. This relocalization after chronic antidepressant treatment has not been observed with other G protein α subunits (Donati et al, 2001; Toki et al, 1999) and the total cellular content of G protein is not altered by antidepressant treatment (Chen and Rasenick, 1995a; Emamghoreishi et al, 1996; Toki et al, 1999).
Recently, the lipid environment in which G proteins and their effectors are localized has been under investigation (Ostrom and Insel, 2004). G proteins appear to be present in both caveolae and cholesterol/sphingolipid-enriched plasma membrane raft domains (Oh and Schnitzer, 2001), and caveolin may play a role in mediating G protein signaling (Bhatnagar et al, 2004; Li et al, 1995). While both Gsα and Giα can move in and out of caveolae, they are predominantly found in lipid rafts complexed with Gβγ (Oh and Schnitzer, 2001). The direct association of G proteins with caveolin has been disputed (Huang et al, 1997); however, these authors conclude that the proteins involved in the hormone-sensitive adenylyl cyclase system are indeed localized to a specialized subdomain of the plasma membrane. Figure 3 clearly shows that many of the proteins involved in the Gsα signaling cascade are localized in the caveolae/lipid raft-enriched TIFFs. The cholesterol chelating drug, methyl-β-cyclodextrin, can be used to disrupt these cholesterol-rich membrane domains and study the proteins localized within them (Miura et al, 2001; Nichols, 2003). With the exception of adenylyl cyclase V/VI, all of the other proteins shown in Figure 3 and Table 1 have a decreased TIFF localization after cholesterol depletion, indicating the importance of these domains as scaffolds to concentrate signaling molecules. While Gsα is extremely sensitive to the loss of cholesterol, chronic desipramine treatment does not affect the cholesterol content of the membrane (Figure 2) nor the raft localization of these proteins, with the exception of Gsα (Figure 3). This suggests that the antidepressant-induced disruption of Gsα from TIFFs is not due to any direct effect of these drugs on cholesterol or cholesterol-dependent membrane structure. Rather, it appears that chronic antidepressant treatment directly disrupts the association between Gsα and some other molecule(s) that target Gsα to TIFFs.
Studies by Miyamoto et al (1997) revealed that chronic, but not acute treatment of rats with desipramine resulted in decreased microtubule assembly and nucleation in the cerebral cortex. Chronic desipramine treatment has also been reported to phosphorylate certain microtubule-associated proteins (Perez et al, 1989). A fraction of PKA II associates with microtubules (Perez et al, 1993; Theurkauf and Vallee, 1982) via MAP2, which serves as an anchor and substrate for PKA II (Obar et al, 1989; Rubino et al, 1989). Thus, chronic PKA II activation as seen during antidepressant treatment leads to MAP2 phosphorylation and a subsequent decrease in microtubule assembly (Perez et al, 2000).
The decrease in microtubule assembly and nucleation due to increased PKA II activity seen after chronic antidepressant treatment (Perez et al, 2000) should lead to increased tubulin dimers within the cell. Tubulin has been shown to interact directly with Gsα (Wang and Rasenick, 1991; Wang et al, 1990; Yan et al, 1996, 2001) and transfer GTP to G protein α subunits. Experiments with a GTP photoaffinity analog suggest that tubulin dimers form complexes with the α subunits of Gs, Gi1, or Gq and activate them through a direct nucleotide transfer (transactivation) mechanism (Popova and Rasenick, 2000; Rasenick and Wang, 1988; Roychowdhury et al, 1993; Yan et al, 2001). Antidepressants may increase the pool of available tubulin dimers leading to the sustained transactivation of Gsα and subsequent association of that Gsα with adenylyl cyclase in a receptor-independent manner. This would allow increased cAMP formation even in the presence of a downregulation of receptors normally coupled to Gsα. Thus, microtubule-disrupting agents should have antidepressant-like effects on Gsα TIFF localization by increasing the pool of tubulin dimers capable of transactivation. Figure 4 demonstrates that colchicine does in fact decrease the amount of Gsα in the TIFF fraction comparable to the extent seen with desipramine treatment.
Alternatively, or in conjunction with the above hypothesis, chronic desipramine treatment may lead to a release of the microtubule tether hindering the coupling of Gsα with adenylyl cyclase. It is well established that treatment of cells with microtubule-disrupting agents like colchicine and vinblastine leads to an increase in G protein-mediated activation of adenylyl cyclase (Kennedy and Insel, 1979; Rasenick et al, 1981, 1984; Leiber et al, 1993; Jasper et al, 1995; Nishigaki et al, 1998; Rudolph et al, 1979). These potential actions would allow the cAMP signal to be propagated after receptor desensitization mediated by antidepressant treatment (reviewed by Sulser, 1984). Recent evidence has shown that it is the duration of cAMP expression rather than the quantity of the cAMP increase that is important for the downstream activation of CREB-mediated gene transcription (Baker et al, 2004), and there is evidence that chronic antidepressant treatment leads to an increase in the amount of CREB and phospho-CREB in the cell (Duman et al, 1997; Nibuya et al, 1996; Takahashi et al, 1999; Thome et al, 2000).
Previous studies have suggested that depletion of serum cholesterol in both humans and non-human primates leads to depressive symptoms (Kaplan et al, 1994; Steegmans et al, 2000; Golomb et al, 2004), although this is not a universal finding (Yang et al, 2003). These data, at first glance, appear to contradict the findings that agents such as cyclodextrin that chelate membrane cholesterol have similar effects on Gsα localization as chronic antidepressant treatment. It must be noted, however, that the cyclodextrin effect in this study is on cellular (total and membrane) and not serum cholesterol. Furthermore, the antidepressant drugs tested in this study had no effect on either membrane or total cellular cholesterol. Thus, it is possible that effects of lowered serum cholesterol on mood may have been due to altered levels of steroid cholesterol metabolites.
The structure and function of caveolae and lipid rafts in the nervous system is beginning to be elucidated (Maekawa et al, 2003; Masserini et al, 1999; Tsui-Pierchala et al, 2002) and these structures may become important in defining neurological and psychiatric disease. Data in this report clearly demonstrate that Gsα raft localization is altered by chronic antidepressant treatment, and that this may be due to an alteration of the membrane-associated microtubule cytoskeleton. These data are consistent with the suggestion that chronic antidepressant treatment ultimately leads to an increase in CREB-mediated gene transcription and BDNF signaling. If we couple the recent findings implicating lipid rafts in the maintenance of synaptic density and morphology in rat primary hippocampal neurons (Hering et al, 2003) with those demonstrating that chronic rolipram (a phosphodiesterase inhibitor) treatment increases hippocampal neuritogenesis in mice (Nakagawa et al, 2002), it appears that lipid rafts may be important in the formation and maintenance of the synapses of these newly formed neurites. Data from this report clearly show that chronic antidepressant treatment does not alter the cholesterol content of the plasma membrane, only the content of Gsα in lipid rafts, and therefore would not hinder, and may enhance synapse formation in these new neurons. Further study on the G protein/raft/cytoskeleton relationship may help to establish relationships between cell structure and G protein signaling and may also lead to an increased understanding of the cellular basis of mood disorders.
References
Allen JA, Yu J-Z, Donati RJ, Rasenick MM (2004). β-Adrenergic receptor stimulation promotes Gαs internalization from lipid rafts. Mol Pharmacol (in press).
Baker JG, Hall IP, Hill SJ (2004). Temporal characteristics of cAMP response element-mediated gene transcription: requirement for sustained cAMP production. Mol Pharmacol 65: 986–998.
Bayewitch ML, Nevo I, Avidor-Reiss T, Levy R, Simonds WF, Vogel Z (2000). Alterations in detergent solubility of heterotrimeric G proteins after chronic activation of G(i/o)-coupled receptors: changes in detergent solubility are in correlation with onset of adenylyl cyclase superactivation. Mol Pharmacol 57: 820–825.
Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL (2004). Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem 279: 34614–34623.
Brown DA, London E (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275: 17221–17224.
Chen J, Rasenick MM (1995a). Chronic treatment of C6 glioma cells with antidepressant drugs increases functional coupling between a G protein (Gs) and adenylyl cyclase. J Neurochem 64: 724–732.
Chen J, Rasenick MM (1995b). Chronic antidepressant treatment facilitates G protein activation of adenylyl cyclase without altering G protein content. J Pharmacol Exp Ther 275: 509–517.
De Montis GM, Devoto P, Gessa GL, Porcella A, Serra G, Tagliamonte A (1990). Selective adenylate cyclase increase in the limbic area of long-term imipramine-treated rats. Eur J Pharmacol 180: 169–174.
Donati RJ, Thukral C, Rasenick MM (2001). Chronic treatment of C6 glioma cells with antidepressant drugs results in a redistribution of Gsalpha. Mol Pharmacol 59: 1426–1432.
Duman RS, Heninger GR, Nestler EJ (1997). A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 597–606.
Emamghoreishi M, Warsh JJ, Sibony D, Li PP (1996). Lack of effect of chronic antidepressant treatment on Gs and Gi alpha-subunit protein and mRNA levels in the rat cerebral cortex. Neuropsychopharmacology 15: 281–287.
Golomb BA, Kane T, Dimsdale JE (2004). Severe irritability associated with statin cholesterol-lowering drugs. Q J Med 97: 229–235.
Hering H, Lin C-C, Sheng M (2003). Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci 23: 3262–3271.
Hough C, Fukamauchi F, Chuang DM (1994). Regulation of beta-adrenergic receptor mRNA in rat C6 glioma cells is sensitive to the state of microtubule assembly. J Neurochem 62: 421–430.
Huang C, Hepler JR, Chen LT, Gilman AG, Anderson RG, Mumby SM (1997). Organization of G proteins and adenylyl cyclase at the plasma membrane. Mol Biol Cell 8: 2365–2378.
Jasper JR, Post SR, Desai KH, Insel PA, Bernstein D (1995). Colchicine and cytochalasin B enhance cyclic AMP accumulation via postreceptor actions. J Pharmacol Exp Ther 274: 937–942.
Kamada H, Saito T, Hatta S, Toki S, Ozawa H, Watanabe M et al (1999). Alterations of tubulin function caused by chronic antidepressant treatment in rat brain. Cell Mol Neurobiol 19: 109–117.
Kaplan JR, Shively CA, Fontenot MB, Morgan TM, Howell SM, Manuck SB et al (1994). Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med 56: 479–484.
Kennedy M, Insel P (1979). Inhibitor of microtubule assembly enhance beta-adrenergic and prostaglandin E1-stimulated cyclic AMP accumulation in S49 lymphoma cells. Mol Pharmacol 16: 215.
Kuo SC, Sheetz MP (1993). Force of single kinesin molecules measured with optical tweezers. Science 260: 232–234.
Leiber D, Jasper JR, Alousi AA, Martin J, Bernstein D, Insel PA (1993). Alteration in Gs-mediated signal transduction in S49 lymphoma cells treated with inhibitors of microtubules. J Biol Chem 268: 3833–3837.
Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH et al (1995). Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem 270: 15693–15701.
Maekawa S, Iino S, Miyata S (2003). Molecular characterization of the detergent-insoluble cholesterol-rich membrane microdomain (raft) of the central nervous system. Biochim Biophys Acta 1610: 261–270.
Masserini M, Palestini P, Pitto M (1999). Glycolipid-enriched caveolae and caveolae-like domains in the nervous system. J Neurochem 73: 1–11.
Menkes DB, Rasenick MM, Wheeler MA, Bitensky MW (1983). Guanosine triphosphate activation of brain adenylate cyclase: enhancement by long-term antidepressant treatment. Science 219: 65–67.
Miura Y, Hanada K, Jones TL (2001). G(s) signaling is intact after disruption of lipid rafts. Biochemistry 40: 15418–15423.
Miyamoto S, Asakura M, Sasuga Y, Osada K, Bodaiji N, Imafuku J et al (1997). Effects of long-term treatment with desipramine on microtubule proteins in rat cerebral cortex. Eur J Pharmacol 333: 279–287.
Moffett S, Brown DA, Linder ME (2000). Lipid-dependent targeting of G proteins into rafts. J Biol Chem 275: 2191–2198.
Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C et al (2002). Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 22: 3673–3682.
Neubig RR (1994). Membrane organization in G-protein mechanisms. FASEB J 8: 939–946.
Nibuya M, Nestler EJ, Duman RS (1996). Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16: 2365–2372.
Nichols B (2003). Caveosomes and endocytosis of lipid rafts. J Cell Sci 116: 4707–4714.
Nishigaki N, Chang C, Ichikawa A, Negishi M (1998). Cytoskeletal regulation of the signal transduction of prostaglandin EP4 receptor. Biochim Biophys Acta 1391: 110–116.
Obar RA, Dingus J, Bayley H, Vallee RB (1989). The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron 3: 639–645.
Oh P, Schnitzer JE (2001). Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell 12: 685–698.
Ostrom RS, Insel PA (2004). The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol 143: 235–245.
Ozawa H, Rasenick MM (1989). Coupling of the stimulatory GTP-binding protein Gs to rat synaptic membrane adenylate cyclase is enhanced subsequent to chronic antidepressant treatment. Mol Pharmacol 36: 803–808.
Ozawa H, Rasenick MM (1991). Chronic electroconvulsive treatment augments coupling of the GTP-binding protein Gs to the catalytic moiety of adenylyl cyclase in a manner similar to that seen with chronic antidepressant drugs. J Neurochem 56: 330–338.
Perez J, Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R (2000). Abnormalities of cAMP signaling in affective disorders: implication for pathophysiology and treatment. Bipolar Disord 2: 27–36.
Perez J, Tinelli D, Bianchi E, Brunello N, Racagni G (1991). cAMP binding proteins in the rat cerebral cortex after administration of selective 5-HT and NE reuptake blockers with antidepressant activity. Neuropsychopharmacology 4: 57–64.
Perez J, Tinelli D, Brunello N, Racagni G (1989). cAMP-dependent phosphorylation of soluble and crude microtubule fractions of rat cerebral cortex after prolonged desmethylimipramine treatment. Eur J Pharmacol 172: 305–316.
Perez J, Tinelli D, Cagnoli C, Pecin P, Brunello N, Racagni G (1993). Evidence for the existence of cAMP-dependent protein kinase phosphorylation system associated with specific phosphoproteins in stable microtubules from rat cerebral cortex. Brain Res 602: 77–83.
Popova JS, Rasenick MM (2000). Muscarinic receptor activation promotes the membrane association of tubulin for the regulation of Gq-mediated phospholipase Cbeta(1) signaling. J Neurosci 20: 2774–2782.
Rasenick MM, Donati RJ, Popova JS, Yu J-Z (2004). Tubulin as a regulator of G protein signaling. Methods in Enzymology 390: 389–403.
Rasenick MM, Kaplan RS (1986). Guanine nucleotide activation of adenylate cyclase in saponin permeabilized glioma cells. FEBS Lett 207: 296–301.
Rasenick MM, Stein PJ, Bitensky M (1981). The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature 294: 560–562.
Rasenick MM, Wang N (1988). Exchange of guanine nucleotides between tubulin and GTP-binding proteins that regulate adenylate cyclase: cytoskeletal modification of neuronal signal transduction. J Neurochem 51: 300–311.
Rasenick MM, Wheeler GL, Bitensky MW, Kosack CM, Malina RL, Stein PJ (1984). Photoaffinity identification of colchicine-solubilized regulatory subunit from rat brain adenylate cyclase. J Neurochem 43: 1447–1454.
Roychowdhury S, Wang N, Rasenick MM (1993). G protein binding and G protein activation by nucleotide transfer involve distinct domains on tubulin: regulation of signal transduction by cytoskeletal elements. Biochemistry 32: 4955–4961.
Rubino HM, Dammerman M, Shafit-Zagardo B, Erlichman J (1989). Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase on MAP2. Neuron 3: 631–638.
Rudolph SA, Hegstrand LR, Greengard P, Malawista S (1979). The interaction of colchicine with hormone-sensitive adenylate cyclase in human leukocytes. Mol Pharmacol 16: 805.
Sarma T, Voyno-Yasenetskaya T, Hope TJ, Rasenick MM (2003). Heterotrimeric G-Proteins associate with micro tubules during differentiation in PC12 pheochromocytoma cells. FASEB J 17: 848–859.
Steegmans PHA, Hoes AW, Bak AA, Van der Does E, Grobbee DE (2000). Higher prevalence of depressive symptoms in middle-aged men with low serum cholesterol levels. Psychsom Med 62: 205–211.
Sulser F (1984). Antidepressant treatments and regulation of norepinephrine-receptor-coupled adenylate cyclase systems in brain. Adv Biochem Psychopharmacol 39: 249–261.
Takahashi M, Terwilliger R, Lane C, Mezes PS, Conti M, Duman RS (1999). Chronic antidepressant administration increases the expression of cAMP-specific phosphodiesterase 4A and 4B isoforms. J Neurosci 19: 610–618.
Theurkauf WE, Vallee RB (1982). Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem 257: 3284–3290.
Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S et al (2000). cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci 20: 4030–4036.
Toki S, Donati RJ, Rasenick MM (1999). Treatment of C6 glioma cells and rats with antidepressant drugs increases the detergent extraction of G(s alpha) from plasma membrane. J Neurochem 73: 1114–1120.
Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson Jr EM (2002). Lipid rafts in neuronal signaling and function. Trends Neurosci 25: 412–417.
Wang N, Rasenick MM (1991). Tubulin–G protein interactions involve microtubule polymerization domains. Biochemistry 30: 10957–10965.
Wang N, Yan K, Rasenick MM (1990). Tubulin binds specifically to the signal-transducing proteins, Gsa and Gia1. J Biol Chem 265: 1239–1242.
Wong DT, Bymaster FP, Engleman EA (1995). Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci 57: 411–441.
Yan K, Greene E, Belga F, Rasenick MM (1996). Synaptic membrane G proteins are complexed with tubulin in situ. J Neurochem 66: 1489–1495.
Yan K, Popova JS, Moss A, Shah B, Rasenick MM (2001). Tubulin stimulates adenylyl cyclase activity in C6 glioma cells by bypassing the ss-adrenergic receptor: a potential mechanism of G protein activation. J Neurochem 76: 182–190.
Yang C-C, Jick SS, Jick H (2003). Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med 163: 1926–1932.
Acknowledgements
We thank Dr Brian Layden for critical review of this manuscript and Ms Bindu Shah for help with the cholesterol studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donati, R., Rasenick, M. Chronic Antidepressant Treatment Prevents Accumulation of Gsα in Cholesterol-Rich, Cytoskeletal-Associated, Plasma Membrane Domains (Lipid Rafts). Neuropsychopharmacol 30, 1238–1245 (2005). https://doi.org/10.1038/sj.npp.1300697
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300697
Keywords
This article is cited by
-
A novel peripheral biomarker for depression and antidepressant response
Molecular Psychiatry (2022)
-
Potential depression and antidepressant-response biomarkers in human lymphoblast cell lines from treatment-responsive and treatment-resistant subjects: roles of SSRIs and omega-3 polyunsaturated fatty acids
Molecular Psychiatry (2021)
-
Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds
Neuropsychopharmacology (2018)
-
Regulation of monoamine transporters and receptors by lipid microdomains: implications for depression
Neuropsychopharmacology (2018)
-
Prolonged Morphine Treatment Alters Expression and Plasma Membrane Distribution of β-Adrenergic Receptors and Some Other Components of Their Signaling System in Rat Cerebral Cortex
Journal of Molecular Neuroscience (2017)