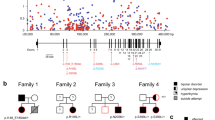
Abstract
Many bipolar affective disorder (BD) susceptibility loci have been identified but the molecular mechanisms responsible for the disease remain to be elucidated. In the locus 4p16, several candidate genes were identified but none of them was definitively shown to be associated with BD. In this region, the PPP2R2C gene encodes the Bγ-regulatory subunit of the protein phosphatase 2A (PP2A-Bγ). First, we identified, in two different populations, single nucleotide polymorphisms and risk haplotypes for this gene that are associated to BD. Then, we used the Bγ subunit as bait to screen a human brain cDNA library with the yeast two-hybrid technique. This led us to two new splice variants of KCNQ2 channels and to the KCNQ2 channel itself. This unusual K+ channel has particularly interesting functional properties and belongs to a channel family that is already known to be implicated in several other monogenic diseases. In one of the BD populations, we also found a genetic association between the KCNQ2 gene and BD. We show that KCNQ2 splice variants differ from native channels by their shortened C-terminal sequences and are unique as they are active and exert a dominant-negative effect on KCNQ2 wild-type (wt) channel activity. We also show that the PP2A-Bγ subunit significantly increases the current generated by KCNQ2wt, a channel normally inhibited by phosphorylation. The kinase glycogen synthase kinase 3 beta (GSK3β) is considered as an interesting target of lithium, the classical drug used in BD. GSK3β phosphorylates the KCNQ2 channel and this phosphorylation is decreased by Li+.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Goodwin FK . From the alcohol, drug abuse, and mental health administration. JAMA 1990; 264: 2495.
Blackwood DH, He L, Morris SW, McLean A, Whitton C, Thomson M et al. A locus for bipolar affective disorder on chromosome 4p. Nat Genet 1996; 12: 427–430.
Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G et al. A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc Natl Acad Sci USA 1999; 96: 5604–5609.
Polymeropoulos MH, Schaffer AA . Scanning the genome with 1772 microsatellite markers in search of a bipolar disorder susceptibility gene. Mol Psychiatr 1996; 1: 404–407.
Ewald H, Degn B, Mors O, Kruse TA . Support for the possible locus on chromosome 4p16 for bipolar affective disorder. Mol Psychiatr 1998; 3: 442–448.
Als TD, Dahl HA, Flint TJ, Wang AG, Vang M, Mors O et al. Possible evidence for a common risk locus for bipolar affective disorder and schizophrenia on chromosome 4p16 in patients from the Faroe Islands. Mol Psychiatr 2004; 9: 93–98.
Swift RG, Polymeropoulos MH, Torres R, Swift M . Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol Psychiatr 1998; 3: 86–91.
Kato T, Iwamoto K, Washizuka S, Mori K, Tajima O, Akiyama T et al. No association of mutations and mRNA expression of WFS1/wolframin with bipolar disorder in humans. Neurosci Lett 2003; 338: 21–24.
Abou Jamra R, Schumacher J, Golla A, Richter C, Otte AC, Schulze TG et al. Family-based association studies of alpha-adrenergic receptor genes in chromosomal regions with linkage to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet 2004; 126: 79–81.
Kirov G, Jones I, McCandless F, Craddock N, Owen MJ . Family-based association studies of bipolar disorder with candidate genes involved in dopamine neurotransmission: DBH, DAT1, COMT, DRD2, DRD3 and DRD5. Mol Psychiatr 1999; 4: 558–565.
Muir WJ, Thomson ML, McKeon P, Mynett-Johnson L, Whitton C, Evans KL et al. Markers close to the dopamine D5 receptor gene (DRD5) show significant association with schizophrenia but not bipolar disorder. Am J Med Genet 2001; 105: 152–158.
Strack S, Zaucha JA, Ebner FF, Colbran RJ, Wadzinski BE . Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol 1998; 392: 515–527.
Trojanowski JQ, Lee VM . Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J 1995; 9: 1570–1576.
Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG . An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005; 122: 261–273.
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 2002; 99: 13675–13680.
Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci 2003; 6: 564–571.
Gargus JJ . Unraveling monogenic channelopathies and their implications for complex polygenic disease. Am J Hum Genet 2003; 72: 785–803.
Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ et al. A potassium channel mutation in neonatal human epilepsy. Science 1998; 279: 403–406.
Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet 1998; 18: 25–29.
Biervert C, Steinlein OK . Structural and mutational analysis of KCNQ2, the major gene locus for benign familial neonatal convulsions. Hum Genet 1999; 104: 234–240.
Maljevic S, Lerche C, Seebohm G, Alekov AK, Busch AE, Lerche H . C-terminal interaction of KCNQ2 and KCNQ3 K+ channels. J Physiol 2003; 548: 353–360.
Schwake M, Jentsch TJ, Friedrich T . A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep 2003; 4: 76–81.
Nakajo K, Kubo Y . Protein kinase C shifts the voltage dependence of KCNQ/M channels expressed in Xenopus oocytes. J Physiol 2005; 569: 59–74.
Bhat RV, Budd Haeberlein SL, Avila J . Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem 2004; 89: 1313–1317.
O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci 2004; 24: 6791–6798.
Kennelly PJ, Krebs EG . Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 1991; 266: 15555–15558.
Schroeder BC, Kubisch C, Stein V, Jentsch TJ . Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 1998; 396: 687–690.
Visscher PM, Haley CS, Ewald H, Mors O, Egeland J, Thiel B et al. Joint multi-population analysis for genetic linkage of bipolar disorder or ‘wellness’ to chromosome 4p. Am J Med Genet B Neuropsychiatr Genet 2005; 133: 18–24.
Janssens V, Goris J . Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 2001; 353: 417–439.
Meyre D, Bouatia-Naji N, Tounian A, Samson C, Lecoeur C, Vatin V et al. Variants of ENPP1 are associated with childhood and adult obesity and increase the risk of glucose intolerance and type 2 diabetes. Nat Genet 2005; 37: 863–867.
Tinel N, Lauritzen I, Chouabe C, Lazdunski M, Borsotto M . The KCNQ2 potassium channel: splice variants, functional and developmental expression. Brain localization and comparison with KCNQ3. FEBS Lett 1998; 438: 171–176.
Tinel N, Diochot S, Lauritzen I, Barhanin J, Lazdunski M, Borsotto M . M-type KCNQ2-KCNQ3 potassium channels are modulated by the KCNE2 subunit. FEBS Lett 2000; 480: 137–141.
Schmidt K, Kins S, Schild A, Nitsch RM, Hemmings BA, Gotz J . Diversity, developmental regulation and distribution of murine PR55/B subunits of protein phosphatase 2A. Eur J Neurosci 2002; 16: 2039–2048.
Weber YG, Geiger J, Kampchen K, Landwehrmeyer B, Sommer C, Lerche H . Immunohistochemical analysis of KCNQ2 potassium channels in adult and developing mouse brain. Brain Res 2006; 1077: 1–6.
Chouabe C, Neyroud N, Guicheney P, Lazdunski M, Romey G, Barhanin J . Properties of KvLQT1 K+ channel mutations in Romano–Ward and Jervell and Lange–Nielsen inherited cardiac arrhythmias. EMBO J 1997; 16: 5472–5479.
Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet 1996; 12: 17–23.
Jentsch TJ, Schroeder BC, Kubisch C, Friedrich T, Stein V . Pathophysiology of KCNQ channels: neonatal epilepsy and progressive deafness. Epilepsia 2000; 41: 1068–1069.
Jentsch TJ . Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 2000; 1: 21–30.
Avramopoulos D, Willour VL, Zandi PP, Huo Y, MacKinnon DF, Potash JB et al. Linkage of bipolar affective disorder on chromosome 8q24: follow-up and parametric analysis. Mol Psychiatr 2004; 9: 191–196.
Pan Z, Selyanko AA, Hadley JK, Brown DA, Dixon JE, McKinnon D . Alternative splicing of KCNQ2 potassium channel transcripts contributes to the functional diversity of M-currents. J Physiol 2001; 531: 347–358.
Xie X, Hagan RM . Cellular and molecular actions of lamotrigine: possible mechanisms of efficacy in bipolar disorder. Neuropsychobiology 1998; 38: 119–130.
Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D . Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci 2005; 8: 51–60.
Quiroz JA, Gould TD, Manji HK . Molecular effects of lithium. Mol Interv 2004; 4: 259–272.
Suh BC, Hille B . Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 2002; 35: 507–520.
Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T et al. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron 2003; 37: 963–975.
Delmas P, Brown DA . Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 2005; 6: 850–862.
Klein PS, Melton DA . A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 1996; 93: 8455–8459.
Cooper EC, Jan LY . M-channels: neurological diseases, neuromodulation, and drug development. Arch Neurol 2003; 60: 496–500.
Peretz A, Degani N, Nachman R, Uziyel Y, Gibor G, Shabat D et al. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 2005; 67: 1053–1066.
Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA et al. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci 2003; 23: 7227–7236.
Cavarec L, Kamphausen T, Dubourg B, Callebaut I, Lemeunier F, Metivier D et al. Identification and characterization of Moca-cyp. A Drosophila melanogaster nuclear cyclophilin. J Biol Chem 2002; 277: 41171–41182.
Acknowledgements
We are very grateful to Dr Thomas Jentsch for the generous gift of the KCNQ2wt channels and Dr Gary Buell for helpful discussion. We thank Martine Jodar, Nathalie Leroudier, Aurélie Kiers and Franck Aguila for their expert technical assistance, Linda Belo for reading the manuscript and Yvette Benhamou for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Duality of Interest
None declared.
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website (http://www.nature.com/tpj)
Supplementary information
Rights and permissions
About this article
Cite this article
Borsotto, M., Cavarec, L., Bouillot, M. et al. PP2A-Bγ subunit and KCNQ2 K+ channels in bipolar disorder. Pharmacogenomics J 7, 123–132 (2007). https://doi.org/10.1038/sj.tpj.6500400
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.tpj.6500400
Keywords
This article is cited by
-
GSK-3β Disrupts Neuronal Oscillatory Function to Inhibit Learning and Memory in Male Rats
Cellular and Molecular Neurobiology (2022)
-
Protein Phosphatase 2A: a Double-Faced Phosphatase of Cellular System and Its Role in Neurodegenerative Disorders
Molecular Neurobiology (2018)
-
Autism spectrum disorder and epileptic encephalopathy: common causes, many questions
Journal of Neurodevelopmental Disorders (2017)
-
Modulation of Kv7 channels and excitability in the brain
Cellular and Molecular Life Sciences (2017)
-
Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1
BMC Medical Genetics (2014)