Abstract
Aggregation of alpha synuclein (α-syn) leading to dopaminergic neuronal death has been recognized as one of the main pathogenic factors in the initiation and progression of Parkinson’s disease (PD). Consequently, α-syn has been targeted for the development of therapeutics for PD. We have developed a novel assay to screen compounds with α-syn modulating properties by mimicking recent findings from in vivo animal studies involving intrastriatal administration of pre-formed fibrils in mice, resulting in increased α-syn pathology accompanying the formation of Lewy-body (LB) type inclusions. We found that in vitro generated α-syn pre-formed fibrils induce seeding of α-syn monomers to produce aggregates in a dose-and time-dependent manner under static conditions in vitro. These aggregates were toxic towards rat pheochromocytoma cells (PC12). Our novel multifunctional dopamine agonists D-519 and D-520 exhibited significant neuroprotection in this assay, while their parent molecules did not. The neuroprotective properties of our compounds were further evaluated in a Drosophila model of synucleinopathy. Both of our compounds showed protective properties in fly eyes against the toxicity caused by α-syn. Thus, our in vitro results on modulation of aggregation and toxicity of α-syn by our novel assay were further validated with the in vivo experiments.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, afflicting nearly 1% of the population over the age of 60 years. PD is pathologically characterised by the dopaminergic neuronal loss in the substantia nigra pars compacta and other regions of the brain resulting in the degeneration of the nigro-striatal tract and loss of dopamine (DA)1 and by the presence of Lewy bodies and Lewy neurites consisting primarily of α-synuclein (α-syn) along with Parkin and ubiquitin2. α-Syn is a small, 14-kDa, acidic, natively unfolded protein3, which has been linked to vesicular trafficking, neurotransmitter release, and regulation of neurotransmission4. Evidence from human genetics, animal models, and biochemical and biophysical studies suggests that α-syn aggregation plays a central role in the initiation and/or progression of PD5. Structurally, α-syn can be divided into three regions with distinct structural characteristics6. The N- terminal domain encodes for a series of 11 amino acid imperfect repeats with a consensus motif of KTKEGV, which is capable of forming amphipathic helices7. The central hydrophobic region, also known as the non-amyloid beta component is associated with an increased fibril forming potential8. The acidic C- terminus domain mainly consists of negatively charged residues and is largely unfolded (Fig. 1B).
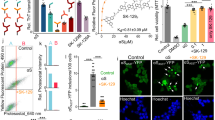
(A) Molecular structures of D-519, D-520, Rifampicin, 5-Hydroxy DPAT and Pramipexole. (B) α-Syn structure and domains: The N terminus domain is shown in white, the non amyloid component domain in dotted region and the C terminus domain in diagonal lines. The KTKEGV imperfect repeats are shown in vertical lines.
Current therapeutic strategies for treating PD mainly offer symptomatic relief by restoring the levels of DA by the administration of levodopa (L-DOPA), a direct precursor of the neurotransmitter. This is used in combination with other drugs like Catechol O-methyl transferase inhibitors (COMTI), monoamine oxidase B inhibitors (MAOBI), and peripheral aromatic L-amino acid decarboxylase inhibitors (AADCI) that help in slowing down the degradation of L-DOPA in the periphery and increasing its availability. However, long term use of L-DOPA gives rise to motor fluctuations with dyskinesias and a decrease in the duration of response time to a given L-DOPA dose9. Moreover, prolonged use of L-DOPA gives rise to “on” and “off” episodes and might lead to toxicity to DA neurons, hence accelerating the DA neurodegeneration process10,11. It has been observed that both L-DOPA and DA generate free radicals during normal metabolism12 and hence the use of dopamine agonists such as Bromocriptine, Pergolide, Ropinirole, and Pramipexole13,14,15,16 have gained a lot of importance for the treatment of PD. However, there is a significant unmet need in the development of symptomatic and disease modifying agents for the treatment of PD.
Due to a lack of thorough understanding of the mechanisms mediating neurodegeneration in PD, the development of therapeutic strategies to block cell death has been limited. However, increasing evidence of a pivotal role of α-syn in the pathogenesis of PD has made it a potential therapeutic target for the development of neuroprotective drugs. The main strategies include the development of anti aggregation compounds, gene silencing approaches and α-syn aggregate clearance strategies17. Out of these, inhibition of α-syn aggregation remains an extremely attractive target from drug development perspective. Some of the inhibitors developed include epigallocatechin-3-gallate (EGCG)18, 3-(1,3-benzodioxol-5-yl)-5-(3-bromophenyl)-1H-pyrazole (anle138b)19, CLR0120 and the prolyl oligopeptidase inhibitor KYP-20479821.
Recombinant α-syn can form filamentous aggregates in vitro similar to those found in the human brain22,23,24,25. However, it has been observed that under static conditions, the generation of fibrils takes several days and the concentration of recombinant protein needed is notably high, between 300 to 500 μM26,27. In order to accelerate this process, several factors have been utilized. These include the use of anions28, polyanions29,30, polycations31, salts28, pesticides and herbicides32,33,34, heavy metals35, negatively charged detergents36, molecular crowding37,38,39, low pH40 and acidic phospholipids41,42. Apart from these factors, agitation has been shown to induce aggregation and several assays have been developed based on this property to screen α-syn aggregation inhibitors43.
Due to the complex pathogenesis of PD, it is recognized that a multifunctional drug addressing the underlying disease processes will be more effective than a drug targeting a single pathological feature of the disease. In our approach, along this line, we designed compounds with both symptomatic and neuroprotective disease modifying effects. Thus, we have designed and biologically characterized multifunctional dopamine agonists D-519 and D-520 as modulators of α-syn aggregation44.
As described above, all the methods for in vitro aggregation of α-syn are intensely aggressive and artificial in the context of its aggregation in the PD brain. In order to overcome this drawback, we have developed a novel assay for the screening of α-syn aggregation inhibitors, wherein monomeric α-syn is seeded with pre-formed fibrils (PFFs) without agitation. It has been shown that α-syn fibrils can seed Lewy body and Lewy neurite like inclusions in the cell culture models22,23. Inoculation of PFFs into mice brain seeds aggregation of the endogenous α-syn and shows neurodegeneration similar to PD24. Similar results have been reported in a rat model recently45. Our novel method was informed by these recent observations.
Our assay, apart from mimicking the conditions that are likely occurring in the PD brain, drastically reduces the time as well as the concentration of α-syn required for aggregation. We observe the formation of aggregates in a time dependent manner. The progression in aggregate formation has been studied by Thioflavin T (ThT) assay, transmission electron microscopy (TEM) and circular dichroism (CD) spectroscopy. We show here that multifunctional dopamine agonists, D-519 and D-520 (Fig. 1A), developed by us are capable of inhibiting α-syn aggregate formation, which resulted in reduction of toxicity. Furthermore, the protective effects of the compounds observed in our novel assay were further confirmed and validated in a GFP-based Drosophila model expressing mutant pathogenic A30P α-syn protein in fly eyes to produce detectable and quantifiable toxicity.
Results
Effect of seeding on the aggregation of monomeric α-syn
The formation of PFFs was confirmed by ThT assay. It was observed that there was nearly a 200 fold increase in the ThT value when compared to monomeric α-syn (Supplementary Fig. 1). To assess the ability of PFFs to induce aggregation of monomeric α-syn, the PFFs at 1% v/v were incubated with 1.25 mg/mL α-syn at 37 °C without agitation for a period of 10 days (D). The degree of fibrillation was measured by ThT assay. It was observed that there was nearly a 20 fold increase in ThT fluorescence value when compared to monomeric α-syn at 0D (Supplementary Fig. 2). However, we wanted the aggregation to take place more gradually in order to mimic the PD conditions. We therefore reduced the concentration of PFFs used for seeding to 0.5% and also increased the incubation time to 30D.
Effect of 0.5% PFFs on the aggregation of monomeric α-syn
Upon incubation of monomeric α-syn with 0.5% PFFs, it was observed that there was a time dependent increase in the aggregation of α-syn as evidenced by ThT fluorescence. There was nearly a 11.6, 18 and 27 fold increase in the ThT value at 10D, 20D and 30D, respectively when compared to monomeric α-syn at 0D (Fig. 2A). Thus, the rate of formation of aggregates as indicated by ThT fluorescence was slower compared to the aggregates formed by 1% PFF seeding at 10D (Supplementary Fig. 2). In order to examine the cytotoxicity of the aggregates formed towards PC12 cells, α-syn aggregates at a concentration of 10 μM were incubated with the cells for a period of 24 H, following which cell viability was measured by MTT assay. It was observed that the aggregates generated from incubation for 30D seeding were most toxic when compared to the corresponding 10D and 20D α-syn seeding samples. The decrease in viability caused by the 0D, 10D, 20D and 30D seeded samples was 23%, 32%, 37% and 48% respectively when compared to the cells treated with monomeric α-syn (Fig. 2B). With this result, we decided to carry out our experiments with 0.5% seeding.
Effect of 0.5% PFF on the aggregation of monomeric α-syn.
1.25 mg/mL α-syn was incubated with 0.5% PFFs for a period of 30D. (A) Fibrillation was measured by ThT assay. Values are represented in terms of % 0D Synuclein. (B) Effect of the aggregates formed by 0.5% seeding on the viability of PC12 cells; PC12 cells were treated with 10 μM aggregates formed for a period of 24 H, following which cell viability was measured by MTT assay. Values are represented in terms of % control. Data values shown are means ± SD of three independent experiments. One-way ANOVA analysis followed by Tukey’s multiple comparison post hoc test was performed, **p ≤ 0.01, ****p ≤ 0.0001 compared to Syn-0D; $$p ≤ 0.01, $$$$p ≤ 0.0001 compared to Syn + 0.5%-0D.
Effect of Rifampicin and the parent compounds 5-OHDPAT and Pramipexole on the aggregation of α-syn with 0.5% seeding
It has been shown that Rifampicin inhibits α-syn fibrillation and disaggregation of fibrils via preferential stabilization of monomeric and soluble oligomeric forms46. α-Syn seeded with 0.5% PFFs was incubated with twice the molar concentration (172.9 μM) of either Rifampicin (reference compound) or the parent compounds Pramipexole or 5-OHDPAT for a period of 30 days. Figure 3A,B,C represent ThT values at 10D, 20D and 30D, respectively. Out of the three compounds, Rifampicin showed a pronounced effect on the inhibition of α-syn aggregation right from 10D until 30D. The decrease in the ThT fluorescence shown by Rifampicin, Pramipexole and 5-OHDPAT at 30D was by 6, 1.5 and 1.7 fold respectively when compared to 30D seeded α-syn (Fig. 3C). The aggregates formed in the presence of these compounds were treated to PC12 cells. Figure 3D,E and F represent the viability of PC12 cells when treated with α-syn aggregates collected at 10D, 20D and 30D respectively. It was observed that the aggregates formed in the presence of Rifampicin, Pramipexole and 5OHDPAT were less toxic when compared to seeded α-syn. The increase in the viability was 44%, 12% and 12% respectively when compared to α-syn seeded with 0.5% PFFs alone at 30D (Fig. 3F).
Effect of reference compound and parent compounds on the aggregation of α-syn induced by seeding with 0.5% PFFs.
1.25 mg/mL α-syn was incubated with 0.5% PFFs for a period of 30D without shaking in the presence of Rifampicin or Pramipexole or 5-OHDPAT at a concentration of 172.9 μM. Fibrillation was measured by ThT assay at 10D (A), 20D (B), 30D (C). Values are represented in terms of % 0D Synuclein. Viability of PC12 cells was measured by MTT assay after 24 h treatment with α-syn seeded samples collected at 10D (D), 20D (E), 30D (F). Values are represented in terms of % control. Data values shown are means ± SD of three independent experiments. One-way ANOVA analysis followed by Tukey’s multiple comparison post hoc test was performed, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 compared to Syn + 0.5%-0D; $p ≤ 0.05, $$p ≤ 0.01, $$$p ≤ 0.001, $$$$p ≤ 0.0001 compared to Syn + 0.5%–10D (A,D) or Syn + 0.5%-20D (B,E) or Syn + 0.5%-30D (C,F).
Effect of multifunctional dopamine D2/D3 agonists D-519 and D-520 on inhibition of aggregation of α-syn
The dopamine D2/D3 agonist compounds D-520 and D-519, which have been synthesized by us, were incubated with α-syn seeded with 0.5% PFFs at twice the molar concentration (172.9 μM) of α-syn for a period of 30D at 37 °C without agitation. Both D-520 and D-519 lead to a significant decrease in the ThT fluorescence (p < 0.0001) when compared to α-syn seeded with 0.5% PFFs alone. This effect was consistent until 30D (Fig. 4A,B,C). There was a 1.7, 2.7 and 4.1 fold increase in the ThT value of the seeded samples at 10D, 20D and 30D respectively, when compared to 0D seeded sample (Fig. 2A). In the presence of our compounds, D-520 and D-519, the ThT value was reduced almost close to the value shown by α-syn alone at 0D, showing that our compounds inhibited α-syn aggregation very efficiently and this effect was consistent until 30D.
Effect of D-520 and D-519 on the aggregation of α-syn induced by seeding with 0.5% PFFs.
1.25 mg/mL α-syn was incubated with 0.5% PFFs for a period of 30D without shaking in the presence of D-520 or D-519 at a concentration of 172.9 μM. Fibrillation was measured by ThT assay at 10D (A), 20D (B), 30D (C). Values are represented in terms of % 0D Synuclein. Viability of PC12 cells was measured by MTT assay after 24 h treatment with α-syn seeded samples collected at 10D (D), 20D (E) and 30D (F). Values are represented in terms of % control. Data values shown are means ± SD of three independent experiments. One-way ANOVA analysis followed by Tukey’s multiple comparison post hoc test was performed, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 compared to Syn + 0.5%-0D; $$$$$p ≤ 0.0001 compared to Syn + 0.5%-10D (A,D) or Syn + 0.5%-20D (B,E) or Syn + 0.5%-30D (C,F).
When PC12 cells were treated with the aggregates formed by 0.5% PFFs seeding, it was observed that the viability went down to 47%, 43% and 32% for 10D, 20D and 30D samples, respectively, compared to control (Fig. 2B). However, the seeded samples formed in the presence of D-520 and D-519 showed less toxicity when compared to their respective time point’s seeded samples without compounds. The viability values of PC12 cells when treated with α-syn seeded samples in the presence of D-520 and D-519 were 84% and 73% at 10D; 88% and 79% at 20D and 90% and 86% at 30D respectively when compared to control (Fig. 4C,D,E).
Morphology of aggregates by Transmission Electron Microscopy
The results from ThT and cytotoxicity data were further corroborated by Transmission Electron Microscopy (TEM). There was a gradual increase in the amount of aggregates formed in the seeded samples. The 0D sample of α-syn seeded with 0.5% PFFs showed large fibrillar structures recruiting the monomers of α-syn. By the end of 30D, these transformed into fibrillar structures which are highly intertwined, complementing the ThT data (Fig. 5A,B,C). In contrast, the compounds D-520 and D-519, lead to the formation of small amorphous aggregates with a few detectable fibrils (Fig. 5D,E,F,G).
Secondary structure changes of α-syn monitored by far UV CD
Figure 6A represents the far-UV CD spectra of α-syn seeded with 0.5% PFFs at various time points. The CD changes are a function of incubation time from 0D to 30D. There is an increase in the intensity of the negative shoulder at about 220 nm and a decrease in the intensity of the negative shoulder at about 203 nm. These observations are consistent with the data showing that the degree of aggregation increases with incubation time47.
In Fig. 6B, the CD spectra of 30D samples have been plotted. It was observed that 30D seeding sample shows an increase in the intensity of the negative shoulder at about 220 nm and a decrease in the intensity of the negative band at about 203 nm representative of a more structured form, similar to the β-peptide backbone structure, when compared to α-syn alone. The presence of compounds D-520 and D-519 showed a somewhat different trend which involved similar absorption behavior to unseeded α-syn at 220 nm but decrease in absorption at 203 nm compared to unseeded and seeded α-syn samples alone.
α-Syn dependent toxicity in Drosophila
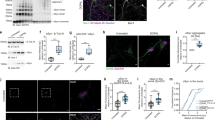
We examined the efficacy of compounds D-519 and D-520 against α-syn-dependent toxicity in a model of synucleinopathy in the fruit fly Drosophila melanogaster by utilizing a highly sensitive and quantitative method which we described recently48. Briefly, this technique relies on a version of GFP that is membrane bound. As eye cells degenerate, GFP fluorescence is lost and can be quantified when imaged through a fluorescent microscope (Figs 7 and 8). The GFP reporter faithfully tracks internal eye degeneration and the technique can be used to identify modifiers of various neurotoxic proteins48,49.
A Drosophila model of α-syn toxicity and the protective effect from Rifampicin.
(A) Representative images of fly eyes not expressing or expressing α-synA30P through the Gal4-UAS system. Driver was GMR-Gal4, which expresses UAS-α-syn in fly eyes. All flies also expressed membrane-targeted GFP (mCD8-GFP), which is independent of the α-syn transgene. Flies were heterozygous for all transgenes. Adult flies were placed in food that contained Rifampicin (1 mg/mL in DMSO) or the vehicle control (DMSO alone) as soon as they emerged from the pupal case and were fed for 28 days, with food changed every three days. Images are from flies that were 28 days old. Dotted lines: Areas quantified in panel B. (B) Quantification of fly eyes from panel A and other flies with the same genotypes. Average GFP fluorescence was normalized to fluorescence from no α-syn, vehicle-treated control flies. *p < 0.05 comparing vehicle treated α-syn eyes to no α-syn column, #p < 0.05 comparing vehicle treated α-syn eyes to Rifampicin treated α-syn eyes, N.S.: statistically not significant, comparing no α-syn eyes to Rifampicin treated α-syn eyes. Values are represented as means ± SD. Statistics: one way ANOVA with Tukey’s post-hoc.
Protective effect from D-519 and D-520 in fly eyes expressing α-syn.
(A) Representative images of flies expressing α-synA30P through the Gal4-UAS system. Flies were fed the vehicle control (ultra pure water) or D-519 or D-520 (1 mg/mL in ultra-pure water) for 28 days. Flies were heterozygous for driver (GMR-Gal4), α-synA30Pand mCD8-GFP. (B) Quantification from fly heads shown in panel A and other flies that were fed vehicle control (ultra-pure water), D-519 or D-520 for the indicated amounts of time. *p < 0.05 based on one way ANOVA with Tukey’s post-hoc comparing flies fed the compound to same-day flies fed with vehicle control. (C) Representative images of fly eyes expressing α-syn and which were fed with either the vehicle control or the indicated compounds at different concentrations dissolved in ultra-pure water. (D) Quantification of eye fluorescence from α-syn-expressing flies that were fed vehicle control, D-519 or D-520 at the indicated concentration for 7 days after the adults emerged from their pupal cases. *p < 0.05 based on one way ANOVA with Tukey’s post-hoc correction comparing compound-fed flies to “ctrl” ones that were fed the vehicle control. Values are represented as means ± SD.
As a proof of principle for the ability of our system to pick up molecular suppressors of α-syn-dependent toxicity, we fed flies Rifampicin (1 mg/mL), a known α-syn aggregation inhibitor. As shown in Fig. 7A (quantified in Fig. 7B), when adult flies are fed Rifampicin for a period of 28 days, fluorescence from α-syn expressing flies is similar to that of eyes which do not express α-syn and are fed with the vehicle alone.
Next, we tested whether the flies fed with D-519 or D-520 (1 mg/mL) for a period of up to 28 days would also display a protective effect. As shown in Fig. 8A and B, over the course of time fluorescence decreases from fly eyes that express α-syn and are not fed with either of the compounds. Both D-519 and D-520 are able to suppress this loss of fluorescence and, over time, even reverse it, indicating a strong protective effect against α-syn-induced toxicity in this in vivo model. When we examined by native gels the type of α-syn species present in this fly model, we found that, concomitant with the increase in GFP intensity in drug-treated flies, there was a decrease in α-syn aggregated species compared to vehicle treated flies (Supplementary Fig. 3).
Treatment with either D-519 or D-520 led to a stronger suppression of phenotype than Rifampicin at 28 days. From analyzing the extent to which treatment by any of these compounds increased GFP fluorescence in fly eyes that express α-syn, Rifampicin led to an increase of ~30% in GFP intensity (Fig. 7B), whereas D-519 and D-520 led to an increase of ~34% and ~57%, respectively (Fig. 8B), in GFP intensity. Based on these results, these compounds are stronger suppressors of toxicity than Rifampicin in Drosophila, which is in agreement with our results from in vitro assays (Figs 3 and 4).
The protective effect of both compounds is dose-dependent, as shown in Fig. 8C and D; higher doses lead to stronger suppression, as early as day 7. Collectively, these results indicate that both D-519 and D-520 are protective against α-syn-dependent toxicity in the fly.
Discussion
α-Syn aggregates have been found in healthy grafted neurons in PD patients and endogenous α-syn has been shown to be recruited into fibrillar aggregates through a seeding mechanism in transgenic mice24,50. These reports laid the foundation for the importance of seeding or nucleation in the aggregation of α-syn. Several in vitro assays for the screening of α-syn aggregation inhibitors have been reported. However, all of these assays are performed under forceful conditions like high speed agitation or under high temperatures, not reflective of actual processes occurring in the human brain. In order to mimic the conditions occurring in the PD brain, here, we have developed a novel in vitro assay for the screening of small molecules which inhibit α-syn aggregation. We applied this assay to assess the activity of our novel multifunctional dopamine agonists D-519 and D-520. The utility of this technique was then validated in an in vivo Drosophila model.
The method which we have developed is without agitation, under “static conditions”. In order to hasten the aggregation, monomeric α-syn is incubated with PFFs which act as seeds to start the nucleation process. To delineate the concentration of PFFs to be used for seeding of monomeric α-syn, we first seeded α-syn with 1% PFFs at 37 °C without agitation for a period of 10D. Seeding with 1% PFFs lead to a 20 fold increase in the ThT value at the end of 10D when compared to monomeric α-syn at 0D (Supplementary Fig. 2). However, we wanted the aggregation to take place more gradually at a slower pace. Hence we decreased the concentration of PFFs used for seeding to 0.5% and also increased the incubation time to 30D. We observed that 0.5% PFFs lead to an increase in the fibrillation by 11.6, 18 and 27 fold at 10D, 20D and 30D, respectively when compared to monomeric α-syn at 0D. However, the sample without seeding showed only a 3 fold increase in fibrillation (Fig. 2A). Our results are supported by previous findings which demonstrated that, the aggregation of α-syn in vitro proceeds in a nucleation dependent process, i.e. fibril formation displays a lag phase followed by an increase in the rate of fibril formation40,51,52. This lag phase has been shown to be reduced significantly by the addition of a seed or a nucleus of pre-aggregated α-syn51,53.
The aggregates formed by seeding at 30D reduced the viability of PC12 cells by 68% as opposed to 44% by the sample generated without seeding (Fig. 2B), strongly indicating pronounced effect of seeding or nucleation dependent effect on aggregation and toxicity (Fig. 2A,B). The reference compound Rifampicin lead to a decrease in the aggregation of seeded α-syn, as measured by ThT fluorescence (Fig. 3A–C) and was also able to decrease the toxicity of the aggregates formed in its presence towards PC12 cells (Fig. 3D–F).
The multifunctional hybrid compounds D-519 and D-520, were derived from parent precursor molecules Pramipexole and 5-OHDPAT by a fragment based drug development approach44,54. The rationale behind testing the parent precursor compounds was to evaluate whether these compounds exhibit an inherent modulation of α-syn aggregation property. Our results indicate that the parent compounds, Pramipexole and 5-OHDPAT, were far less effective in inhibiting aggregation and reducing toxicity of α-syn compared to the reference drug Rifampicin (Fig. 3A–F).
We have previously shown that D-520, a multifunctional dopamine agonist developed in our laboratory is a potent α-syn aggregation inhibitor44. We therefore tested this compound along with its analog D-519 using the assay developed by us. We observed that compared to the ThT activity of seeded α-syn at 30D, D-520 and D-519 induced a 14 fold decrease in ThT activity (Fig. 4C) whereas the reference compound Rifampicin was able to decrease the ThT activity by 6 fold at 30D (Fig. 3C). Also the parent precursor compounds Pramipexole and 5-OHDPAT showed only a 1.5 and 1.7 fold decrease in ThT value, respectively when compared to the seeded α-syn at 30D (Fig. 3C). α-Syn aggregates formed in the presence of D-520 and D-519 were much less toxic towards PC12 cells. The increase in the viabilities were 57% and 53% respectively when compared to the seeded α-syn formed in the absence of the compounds at 30D (Fig. 4D,E,F). This suggests that multifunctional dopamine agonists D-519 and D-520 show a prolonged activity and are efficient even until 30D. In contrast, Rifampicin, Pramipexole and 5-OHDPAT were able to increase the viability by 44%, 12% and 12% respectively when compared to the seeded α-syn formed in the absence of the compounds at 30D (Fig. 3F). This demonstrates that compounds D-520 and D-519 are much better inhibitors to α-syn aggregation and that they also lead to a substantially greater decrease in the toxicity of the aggregates formed in their presence when compared to their parent compounds or the reference compound Rifampicin.
TEM images showed a predominant presence of large fibrillar structures at the end of 30D with seeding (Fig. 5C) with relatively smaller sizes of aggregates at 10D (Fig. 5B). Further analysis by CD spectroscopy revealed the conversion from unordered structures towards a more structured form with an increase in the incubation time as expected (Fig. 6A). However, α-syn samples without seeding did not show any change in the CD spectra data (data not shown). Our analysis of morphologies of aggregates in presence of the drugs by TEM showed that both D-520 and D-519 were able to reduce the aggregation of α-syn and lead to the formation of small amorphous aggregates along with very few fibrils (Fig. 5D–G). In this regard, as shown in Fig. 5D–G and also in the supplemental section (Supplemental Figure 4A–C) the compounds themselves do not form any aggregates. The CD spectra analysis revealed that D-520 and D-519 lead to a decrease in the spectra at 202 nm and an increase in the spectral reading at 222 nm. However, this value was in between that of the unseeded and seeded samples showing that the degree of aggregation has been brought down by our compounds (Fig. 6B). This shows that our compounds are capable of binding to α-syn aggregates, thereby preventing them from further seeding.
It has been shown that expression of wild type and disease-related, mutated variants of α-syn in Drosophila neurons leads to degeneration55. We next evaluated compounds D-520 and D-519 in α-syn dependent toxicity in a model of synucleinopathy in Drosophila (Figs 7 and 8). The fruit fly model was first validated with the positive reference control drug Rifampicin, which suppressed α-syn dependent toxicity in the fly eyes compared to vehicle treated flies (Fig.7A,B). The fact that flies were fed Rifampicin only as adults, not throughout their development, is a testament to the power of this technique and to the efficacy of Rifampicin to suppress α-syn dependent degeneration in Drosophila. We were then able to demonstrate that both D-519 and D-520 rescued fly eyes from toxicity of α-syn over a period of time, from day 7 through 28 (Fig. 8A,B). We also found a concentration dependent protection by D-520, with strongest protection observed with 5 mg/mL concentration (Fig. 8C,D) on day 7. Thus, our results from fly experiments correlate with the data obtained from our new in vitro assay.
In brief, we have been able to demonstrate the development of a novel in vitro assay to screen possible PD therapeutics and to evaluate their effects in inhibiting aggregation of α-syn and toxicity of aggregates. Furthermore, through the utilization of our assay we have been able to demonstrate modulatory properties of α-syn aggregation and toxicity by our novel multifunctional agonists D-520 and D-519. Our results were further validated in an in vivo α-syn dependent toxicity model of synucleinopathy in Drosophila. In the development of our assay, we utilized a novel approach which involved seeding of α-syn with a low concentration of PFFs to initiate the aggregation process by recruiting the monomers to form aggregates. Our approach mimics the recent findings from in vivo animal model study involving intrastriatal administration of PFF in mice and rats resulting in an increased α-syn pathology accompanying the formation of LB type inclusions24,45. Importantly, our approach is quite different from other assays as we do not apply any external forces to induce the aggregation process. Interestingly, we have demonstrated that unlike the multifunctional dopamine agonists D-519 and D-520 developed by us, known dopamine agonists Pramipexole and 5-OHDPAT were much less effective in this assay. The results from our assay were further validated in an in vivo Drosophila synucleinopathy model. The significant protection of fly eyes from toxicity of α-syn by both compounds corresponded well with the results from in vitro assay. Thus, this new assay might offer a more reliable in vitro screening of α-syn inhibitors before advancing to in vivo experiments. Our future studies with more test compounds should further validate the assay.
Materials and Methods
Reagents
Rifamipicin, Thioflavin T, Thiazolyl Blue Tetrazolium Bromide (MTT) were purchased from Sigma Aldrich. Uranyl acetate was purchased from Electron microscopy sciences. Dimethyl sulfoxide (DMSO) and methanol were purchased from Fischer Scientific.
Cell Culture
PC12 Adh (ATCC CRL1721.1) cells, a rat adrenal pheochromocytoma cell line, were purchased from ATCC. RPMI 1640, heat-inactivated horse serum, fetal bovine serum, penicillin-streptomycin, and trypsin were purchased from Gibco. PC12 cells were culture in RPMI 1640 medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum, and 1% Penicillin Streptomycin (100X, 10,000 Units/ml penicillin, 10,000 g/ml streptomycin) at 37 °C in 5% CO2 atmosphere. PC12 cells between the passage numbers 5–15 were used for the experiments.
Purification of α-syn
The plasmid pET28 containing α-syn was a generous gift from Dr. Chad M. Rienstra from University of Illinois. α-Syn purification was carried out according to Huang et al.56. α-Syn was expressed in Escherichia coli BL21(DE3) cells. A single colony was transferred to 50 ml of LB medium containing Kanamycin (30 μg/mL) and incubated at 37 °C overnight. This culture was diluted 100 fold and induced with 100 μM IPTG for a period of 5 h at 37 °C. Osmotic shock was carried out according to Shevchik et al.57. Briefly, the cell pellet from a 1 L culture was resuspended in 100 mL osmotic shock buffer (30 mM Tris–HCl, 40% sucrose, and 2 mM ethylene diamine tetra acetic acid disodium, pH 7.2) and incubated at room temperature for 10 min. The pellet collected by centrifugation at 12,000 rpm for 20 min was resuspended quickly with 90 mL cold water followed by the addition of 37.5 μL of saturated MgCl2, and kept on ice for 3 min. The supernatant containing periplasm proteins was collected by centrifugation at 12,000 rpm for 20 min, and dialyzed against buffer A (20 mM Tris–HCl, pH 8.0) overnight. After centrifugation at 12,000 rpm for 20 min, the supernatant was loaded onto a Hiload 26/10 Q-Sepharose High performance column (GE healthcare) and eluted with a 0–0.5 M NaCl gradient in buffer A. The elution fractions were analyzed by 15% SDS-PAGE, and the fractions containing only 18 kDa band were combined for dialysis against 20 mM NH4HCO3 and further lyophilized. Centrifugation, chromatography, and dialysis were all carried out at 4 °C.
Preparation of α-syn fibrils
Lyophilized α-syn was dissolved in sterile PBS and filtered through a 0.2 μm filter to remove any pre-formed aggregates. α-Syn fibrils were generated by incubating purified α-syn at 5 mg/mL at 37 °C under constant agitation at 1000 rpm in a Thermomix R shaker (Eppendorf, Hamburg, Germany) for a period of 5 days. The fibrils generated were then aliquoted and stored at −80 °C until use23.
Thioflavin T assay
The formation of the fibrils was confirmed by Thioflavin T assay. A volume of 42 μL of 500 mM ThT (1.1 mg of ThT in 7 mL of phosphate-buffered saline (PBS)) solution was mixed with 479 μL of PBS to obtain 40 μM ThT solution. Then 10 μL of protein was mixed with 10 μL of 40 μM ThT in a black 384-well plate (solid bottom, Corning), and the fluorescence was measured using the Synergy Hybrid H1 fluorescence microplate reader (BioTek) at 440 nm excitation and 485 nm emission wavelength with auto sensitivity mode.
Seeding of α-syn monomers with pre-formed fibrils
All the samples were prepared in PBS. Lyophilized α-syn was dissolved in sterile PBS and was filtered through a 0.2 μm filter to remove any pre-formed aggregates. α-Syn at 1.25 mg/mL (86.45 μM) was seeded with either 1% or 0.5% v/v of PFFs at 37 °C without agitation either alone or in the presence of the compounds at twice the molar concentration (172.9 μM) of monomeric α-Syn. Samples were collected at, 10D, 20D and 30D time points. The formation of fibrils was confirmed by ThT assay. (Please refer to supplementary file for a detailed protocol).
Evaluation of cytotoxicity of extracellular α-syn aggregates formed by seeding in cell culture system
PC12 cells were seeded at 17 000 cells/well density in a 96-well plate. 24 h following plating, the cells were treated with α-syn aggregates such that its concentration is 10 μM. 24 h following treatments, viability was measured by MTT assay. MTT at 5 mg/mL was added to the cells such that its volume was 1/10th the volume of the media. Cells were incubated with MTT for a period of 3 h, following which the formazan crystals were solubilized in 100 μL of 1:1 mixture of DMSO/Methanol and the absorbance was measured at 570 and 690 nm using an Epoch microplate reader (BioTek, Winooski, VT). Background-corrected values (570−690 nm) were used to plot the graph. Data from at least three experiments were analyzed using GraphPad software (version 6, San Diego, CA).
Transmission Electron Microscopy
A 4 μL aliquot of α-syn seeding samples was adsorbed onto a Formvar-coated, carbon-stabilized copper grid (400 mesh) for 4 min. The grid was then rinsed briefly with distilled water twice, negatively stained with 2% aqueous uranyl acetate, air-dried, and examined with a JEOL (JEM 2010) transmission electron microscope at an accelerating voltage of 200 kV and 80,000 or 120,000 magnification.
CD measurements
CD spectra were obtained with a Jasco J-1500 CD spectrophotometer. α-Syn samples which were prepared in PBS were diluted to 20 μM in ultrapure water for CD spectra measurements. Spectra were recorded in a 0.01-cm cell from 260 to 190 nm with a step size of 1 nm and a bandwidth of 1 nm. For all spectra, an average of five scans was obtained. CD spectra of the appropriate buffer was recorded and subtracted from the protein spectra.
Drosophila studies
Drosophila stocks were raised and maintained in standard cornmeal media at 25 °C and ~40–60% humidity with 12 h diurnal cycle. For experiments, flies were crossed, raised and maintained at 30 °C in standard media, diurnal cycle and at ~40–60% humidity. Once offspring eclosed from their pupal cases (Day 0), they were switched (on Day 1) to custom-made instant fly food (Genesee Scientific) containing either D-519 or D-520 at a final concentration noted in figures and legends (vehicle was ultra pure water) or Rifampicin (Sigma-Aldrich) at 1 mg/mL (vehicle was DMSO) and maintained at 30 °C. Vehicle control flies were maintained in the same type of food, and at the same conditions.
At specific time points, fly heads were dissected for fluorescence imaging. Dissected heads were imaged using an Olympus BX53 microscope equipped with a DP72 digital camera, and fluorescence from fly eyes was quantified as previously described48,49. All flies in each experimental setup were examined and imaged using the same conditions. UAS-α-syn fly stock was number 8147 from Bloomington Drosophila Stock center. mCD-GFP fly stock was number 892 from Bloomington Drosophila Stock center.
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 6 (GraphPad Software, San Diego, CA, USA). We analyzed all the data by One way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test.
Additional Information
How to cite this article: Yedlapudi, D. et al. Inhibition of alpha-synuclein aggregation by multifunctional dopamine agonists assessed by a novel in vitro assay and an in vivo Drosophila synucleinopathy model. Sci. Rep. 6, 38510; doi: 10.1038/srep38510 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Recchia, A. et al. Alpha-synuclein and Parkinson’s disease. FASEB J 18, 617–626 (2004).
Forno, L. S. Neuropathology of Parkinson’s disease. Journal of Neuropathology and Experimental Neurology 55, 259–272 (1996).
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Clayton, D. F. & George, J. M. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res 58, 120–129 (1999).
Cookson, M. R. Alpha-Synuclein and neuronal cell death. Mol Neurodegener 4, 9 (2009).
Beyer, K. Alpha-Synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol 112, 237–251 (2006).
Davidson, W. S., Jonas, A., Clayton, D. F. & George, J. M. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273, 9443–9449 (1998).
Giasson, B. I., Murray, I. V. J., Trojanowski, J. Q. & Lee, V. M. Y. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 276, 2380–2386 (2001).
Marsden, C. D. & Parkes, J. D. “On-off” effects in patients with Parkinson’s disease on chronic levodopa therapy. Lancet 1, 292–296 (1976).
Fahn, S. Is levodopa toxic? Neurology 47, S184–195 (1996).
Olanow, C. W. Oxidation reactions in Parkinson’s disease. Neurology 40, suppl 32–37; discussion 37–39 (1990).
Asanuma, M., Miyazaki, I. & Ogawa, N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res 5, 165–176 (2003).
Ogawa, N. et al. Bromocriptine protects mice against 6-hydroxydopamine and scavenges hydroxyl free radicals in vitro. Brain Res 657, 207–213 (1994).
Cassarino, D. S., Fall, C. P., Smith, T. S. & Bennett, J. P. Jr. Pramipexole reduces reactive oxygen species production in vivo and in vitro and inhibits the mitochondrial permeability transition produced by the parkinsonian neurotoxin methylpyridinium ion. J Neurochem 71, 295–301 (1998).
Gomez-Vargas, M. et al. Pergolide scavenges both hydroxyl and nitric oxide free radicals in vitro and inhibits lipid peroxidation in different regions of the rat brain. Brain Res 790, 202–208 (1998).
Wu, M., Brudzynski, S. M. & Mogenson, G. J. Differential-Effects of Quinpirole in the Nucleus-Accumbens Depending on the Initial Level of Locomotor-Activity. Brain Res Bull 32, 395–398 (1993).
Dehay, B. et al. Targeting alpha-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14, 855–866 (2015).
Bieschke, J. et al. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA 107, 7710–7715 (2010).
Wagner, J. et al. Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol 125, 795–813 (2013).
Prabhudesai, S. et al. A Novel “Molecular Tweezer” Inhibitor of alpha-Synuclein Neurotoxicity in Vitro and in Vivo. Neurotherapeutics 9, 464–476 (2012).
Myohanen, T. T. et al. A prolyl oligopeptidase inhibitor, KYP-2047, reduces alpha-synuclein protein levels and aggregates in cellular and animal models of Parkinson’s disease. Brit J Pharmacol 166, 1097–1113 (2012).
Luk, K. C. et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proceedings of the National Academy of Sciences of the United States of America 106, 20051–20056 (2009).
Volpicelli-Daley, L. A. et al. Exogenous alpha-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron 72, 57–71 (2011).
Luk, K. C. et al. Pathological alpha-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 338, 949–953 (2012).
Freundt, E. C. et al. Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol 72, 517–524 (2012).
Roostaee, A., Beaudoin, S. & Staskevicius, A. & Roucou, X. Aggregation and neurotoxicity of recombinant alpha-synuclein aggregates initiated by dimerization. Molecular Neurodegeneration 8 (2013).
Conway, K. A. et al. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: Implications for pathogenesis and therapy. P Natl Acad Sci USA 97, 571–576 (2000).
Munishkina, L. A., Fink, A. L. & Uversky, V. N. Accelerated Fibrillation of alpha-Synuclein Induced by the Combined Action of Macromolecular Crowding and Factors Inducing Partial Folding. Curr Alzheimer Res 6, 252–260 (2009).
Cohlberg, J. A., Li, J., Uversky, V. N. & Fink, A. L. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from alpha-synuclein in vitro. Biochemistry-Us 41, 1502–1511 (2002).
Liu, I. H. et al. Agrin binds alpha-synuclein and modulates alpha-synuclein fibrillation. Glycobiology 15, 1320–1331 (2005).
Goers, J., Uversky, V. N. & Fink, A. L. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci 12, 702–707 (2003).
Uversky, V. N., Li, J. & Fink, A. L. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. Febs Lett 500, 105–108 (2001).
Uversky, V. N., Li, J., Bower, K. & Fink, A. L. Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: Implications for Parkinson’s disease. Neurotoxicology 23, 527–536 (2002).
Manning-Bog, A. B. et al. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice - Paraquat and alpha-synuclein. J Biol Chem 277, 1641–1644 (2002).
Uversky, V. N., Li, J. & Fink, A. L. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein - A possible molecular link between Parkinson’s disease and heavy metal exposure. J Biol Chem 276, 44284–44296 (2001).
Giehm, L., Oliveira, C. L. P., Christiansen, G., Pedersen, J. S. & Otzen, D. E. SDS-Induced Fibrillation of alpha-Synuclein: An Alternative Fibrillation Pathway. J Mol Biol 401, 115–133 (2010).
Uversky, V. N., Cooper, E. M., Bower, K. S., Li, J. & Fink, A. L. Accelerated alpha-synuclein fibrillation in crowded milieu. Febs Lett 515, 99–103 (2002).
Munishkina, L. A., Fink, A. L. & Uversky, V. N. Concerted Action of Metals and Macromolecular Crowding on the Fibrillation of alpha-Synuclein. Protein Peptide Lett 15, 1079–1085 (2008).
Shtilerman, M. D., Ding, T. T. & Lansbury, P. T. Molecular crowding accelerates fibrillization of alpha-synuclein: Could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry-Us 41, 3855–3860 (2002).
Uversky, V. N., Li, J. & Fink, A. L. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276, 10737–10744 (2001).
Zhu, M. & Fink, A. L. Lipid binding inhibits alpha-synuclein fibril formation. J Biol Chem 278, 16873–16877 (2003).
Giehm, L., Lorenzen, N. & Otzen, D. E. Assays for alpha-synuclein aggregation. Methods 53, 295–305 (2011).
Zhu, M. et al. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem 279, 26846–26857 (2004).
Modi, G. et al. Multifunctional D2/D3 agonist D-520 with high in vivo efficacy: modulator of toxicity of alpha-synuclein aggregates. ACS Chem Neurosci 5, 700–717 (2014).
Paumier, K. L. et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis 82, 185–199 (2015).
Li, J., Zhu, M., Rajamani, S., Uversky, V. N. & Fink, A. L. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol 11, 1513–1521 (2004).
Serpell, L. C., Berriman, J., Jakes, R., Goedert, M. & Crowther, R. A. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proceedings of the National Academy of Sciences of the United States of America 97, 4897–4902 (2000).
Burr, A. A., Tsou, W. L., Ristic, G. & Todi, S. V. Using membrane-targeted green fluorescent protein to monitor neurotoxic protein-dependent degeneration of Drosophila eyes. J Neurosci Res 92, 1100–1109 (2014).
Tsou, W. L. et al. DnaJ-1 and karyopherin alpha-3 suppress degeneration in a new Drosophila model of Spinocerebellar Ataxia Type 6. Hum Mol Genet (2015).
Hansen, C. et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121, 715–725 (2011).
Narhi, L. et al. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 274, 9843–9846 (1999).
Uversky, V. N., Lee, H. J., Li, J., Fink, A. L. & Lee, S. J. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem 276, 43495–43498 (2001).
Wood, S. J., Wetzel, R., Martin, J. D. & Hurle, M. R. Prolines and amyloidogenicity in fragments of the Alzheimer’s peptide beta/A4. Biochemistry-Us 34, 724–730 (1995).
Dutta, A. K. et al. Synthesis and biological characterization of novel hybrid 7-[[2-(4-phenyl-piperazin-1-yl)-ethyl]-propyl-amino]-5,6,7,8-tetrahydro-naphthale n-2-ol and their heterocyclic bioisosteric analogues for dopamine D2 and D3 receptors. Bioorg Med Chem 12, 4361–4373 (2004).
Feany, M. B. & Bender, W. W. A Drosophila model of Parkinson’s disease. Nature 404, 394–398 (2000).
Huang, C. J., Ren, G. P., Zhou, H. & Wang, C. C. A new method for purification of recombinant human alpha-synuclein in Escherichia coli. Protein Expres Purif 42, 173–177 (2005).
Shevchik, V. E., Condemine, G. & Robertbaudouy, J. Characterization of Dsbc, a Periplasmic Protein of Erwinia-Chrysanthemi and Escherichia-Coli with Disulfide-Isomerase Activity. Embo J 13, 2007–2012 (1994).
Acknowledgements
We are grateful to Dr. Chad M. Rienstra from University of Illinois for a generous gift of the plasmid pET28 for recombinant synthesis of α-syn. We are grateful for the assistance of Drs. Banibrata Das and Seenuvasan Vedachalam in synthesizing D-519 and D-520. We are also grateful to Liping Xu for help with the recombinant synthesis of alpha synuclein protein. We are also grateful to Dr. Timothy Stemmler’s laboratory for help with the use of CD spectrophotometer. This work is supported by National Institute of Neurological Disorders and Stroke/National Institute of Health (NS047198, AKD). This work is also funded by Wayne State President Research Enhancement award (AKD, SVT). The Drosophila fly study is also partially supported by NIH/NINDS (NS 086778, SVT).
Author information
Authors and Affiliations
Contributions
A.K.D. conceived all aspects of the studies including designing of compounds and biological (in vitro and in vivo) experiments reported in the manuscript. D.Y. and A.K.D. designed all alpha synuclein aggregation studies and D.Y. executed all related assays. D.L. and D.Y. carried out T.E.M. and C.D. studies. S.V.T., A.K.D. and G.S.J. designed and G.S.J. and S.V.T. executed fly studies. A.K.D., D.Y. and S.V.T. wrote and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yedlapudi, D., Joshi, G., Luo, D. et al. Inhibition of alpha-synuclein aggregation by multifunctional dopamine agonists assessed by a novel in vitro assay and an in vivo Drosophila synucleinopathy model. Sci Rep 6, 38510 (2016). https://doi.org/10.1038/srep38510
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38510
This article is cited by
-
Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease
Acta Pharmacologica Sinica (2020)
-
Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinson’s disease
Journal of Neural Transmission (2020)
-
Targeting alpha synuclein and amyloid beta by a multifunctional, brain-penetrant dopamine D2/D3 agonist D-520: Potential therapeutic application in Parkinson’s disease with dementia
Scientific Reports (2019)
-
A review on iron chelators as potential therapeutic agents for the treatment of Alzheimer’s and Parkinson’s diseases
Molecular Diversity (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.