-
PDF
- Split View
-
Views
-
Cite
Cite
Esteban A. Fridman, Takashi Hanakawa, Melissa Chung, Friedhelm Hummel, Ramon C. Leiguarda, Leonardo G. Cohen, Reorganization of the human ipsilesional premotor cortex after stroke, Brain, Volume 127, Issue 4, April 2004, Pages 747–758, https://doi.org/10.1093/brain/awh082
Close - Share Icon Share
Abstract
The substrates that mediate recovery of motor function after stroke are incompletely understood. Several primate and human studies proposed the involvement of the premotor cortex of the lesioned hemisphere. Here, we studied four chronic stroke patients with focal subcortical lesions affecting the corticospinal outflow originating in the primary motor cortex (M1) and good motor recovery. We tested the hypothesis that, in these patients, disruption of activity in the premotor cortex of the lesioned hemisphere by transcranial magnetic stimulation (TMS) would result in degraded behaviour in the paretic hand. TMS was applied to the primary motor cortex, dorsal premotor cortex (PMd) and ventral premotor cortex (PMv) of the affected (M1AH, PMdAH, PMvAH) and intact (M1IH, PMdIH, PMvIH) hemispheres of patients and healthy controls in the setting of a simple reaction time (SRT) paradigm performed with the hand contralateral to the stimulated hemisphere. TMS applied to M1 led to substantial contralateral SRT delays in both groups. TMS applied to PMdAH of patients elicited clear delays in contralateral SRT in the paretic hand, whereas TMS applied to PMdIH of patients or healthy volunteers did not. Motor evoked potentials after stimulation of PMdAH were, on average, larger and had, on average, shorter latency than after stimulation of M1AH. These results indicate that PMdAH participates as a substrate mediating functional recovery of executive motor function in patients with focal lesions of corticospinal outflow originating in M1 and good motor recovery. Our results are consistent with the hypothesis that the dorsal premotor cortex of the affected hemisphere can reorganize to control basic parameters of movement usually assigned to M1 function.
Introduction
Stroke is the leading cause of long‐term disability among adults (Dennis et al., 1993; Ferrucci et al., 1993). Functional recovery of motor deficits following ischaemic stroke progresses actively over the initial 4 weeks to 3 months (Kotila et al., 1984; Kelly‐Hayes et al., 1989). Afterward, motor disability remains relatively stable in the absence of specific rehabilitative treatments (Butefisch et al., 1995; Carey et al., 2002) in the majority of patients (Duncan et al., 1992). The clinical features of stroke syndromes depend, among other factors, on lesion sites. For instance, lesions of primary motor cortex (M1) or corticospinal fibres (CS) originating in M1 cause paresis, predominantly of finger movements (Denny‐Brown and Botterell, 1947; Fulton, 1949; Travis, 1955; Fries et al., 1993). Conversely, lesions affecting non‐primary motor areas cause predominantly higher‐order motor disorders such as apraxia (Freund and Hummelsheim, 1985; Halsband and Freund, 1990; Halsband et al., 1993).
The neural substrates underlying motor recovery after chronic stroke are incompletely understood, but it is conceivable that they could differ depending on the location of the lesion and the degree of remaining disability (Rossini et al., 2003). It has been recently reported that the dorsal premotor cortex (PMd) of the intact hemisphere contributes to recovery of motor function in chronic stroke patients with variable degrees of motor disability and variable lesion locations and lesion size in the middle cerebral artery territory (Johansen‐Berg et al., 2002). An important finding of this study was that the contribution of PMd of the intact hemisphere to motor behaviour in the paretic hand was more prominent in patients with greater disability (Johansen‐Berg et al., 2002; Ward et al., 2003).
On the other hand, primate studies proposed that non‐primary motor areas of the affected hemisphere could contribute to functional recovery of the paretic hand following cortical lesions (Seitz et al., 1998; Liu and Rouiller, 1999; Mima et al., 2001; Carey et al., 2002; Frost et al., 2003). Liu and Rouiller (1999), for example, found that muscimol injection of the premotor cortex of the affected, but not the intact hemisphere after recovery from chronic focal lesions of the primary motor cortex, reinstates the motor deficit. Consistent with these findings, Frost et al. demonstrated reorganization in the hand representation of primate ipsilesional premotor cortex associated with functional recovery from focal M1 lesions (Frost et al., 2003). In human studies, recovery of motor function after chronic stroke is also associated with enhanced activation of the PMd of the affected hemisphere (Weiller et al., 1992, 1993; Seitz et al., 1998; Mima et al., 2001; Carey et al., 2002). Therefore, it is conceivable that ipsilesional PMd contributes to the recovery of function in patients with focal interruption of the corticomotor outflow originating in M1 and good motor recovery. The PMd has the properties expected of an area that could assume some of the functions of M1 in the event of a focal stroke: it has neurons that are highly modifiable during motor learning (Wise, 1996; Deiber et al., 1997), pyramidal cells in layer V that project directly to spinal interneurons and alpha motor neurons (Murray and Coulter, 1981; Dum and Strick, 1991, 1996; He et al., 1993), and a somatotopic organization that parallels the organization of M1 (Dum and Strick 1991; He et al., 1993; Wu et al., 2000).
In this study, we evaluated motor performance in selected chronic stroke patients with focal lesions affecting the CS fibres originating in M1 who had an initially severe motor deficit and subsequently experienced substantial motor recovery. We hypothesized that disruption of activity in the premotor cortex of the lesioned hemisphere by transcranial magnetic stimulation (TMS) would result in delayed contralateral simple reaction time (SRT) in the paretic hand. This result would provide evidence supporting the involvement of ipsilesional PMd in stroke recovery.
Methods
Subjects
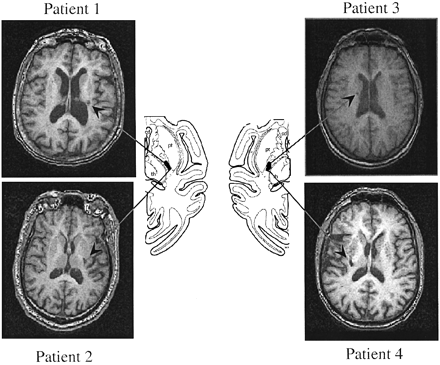
We studied four patients with single clinical ischaemic stroke events dating back >1 year [75.7 ± 1.9 years old; two females and two males, all right‐handed (Oldfield, 1971)] who were initially hemiplegic and, by testing time, had experienced marked motor recovery, 3+ or more on the Medical Research Council (MRC) scale (Medical Research Council, 1976) (see Table 1). Lesions were small focal lacunes in the posterior two‐thirds of the posterior limb of the internal capsule (Fig. 1).
Lesions in this region involve CS fibres originating in M1 but spare CS originating in PMd or ventral premotor cortex (PMv) (Fries et al., 1993; Morecraft et al., 2002). Additional inclusion criteria at testing time included full hand range of motion, absence of spasticity (Ashworth score = 1), and ability to perform index finger flexion and extension. None of the patients had a history of seizures. Five healthy volunteers (67.8 ± 3 years old; two females and three males; all right‐handed) participated as sex‐ and age‐matched controls. All subjects gave their written informed consent according to the Declaration of Helsinki (http://ohsr.od.nih.gov/helsinki.php3), and the NINDS Institutional Review Board approved the study protocol.
Experimental design
SRT paradigm
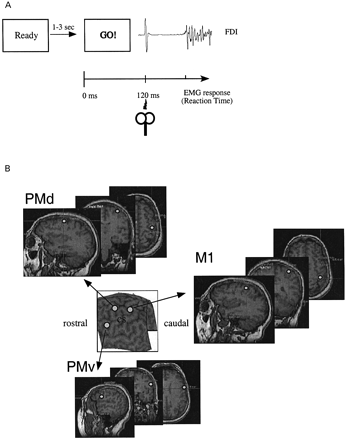
Subjects sat comfortably in a chair with their chins on a chin rest 60 cm from the PC screen, which showed the experimental paradigm displayed with Superlab®. Patients and controls performed an SRT task (Day et al., 1989; Pascual‐Leone et al., 1992a, b; Ziemann et al., 1997). A visually presented warning signal (100 ms duration) was followed at random 1 s to 3.5 s intervals by a GO signal (100 ms duration) (Fig. 2A). Subjects were instructed to respond to the GO signal as quickly as possible by pressing a simple key on a keyboard with the hand contralateral to the stimulated hemisphere. Each experimental session consisted of two initial practice blocks and six test blocks. Blocks were separated by 2 min to avoid fatigue. Each block consisted of 35 trials separated by 8 s intervals: 14 with real TMS; 14 with sham TMS; and seven ‘catch’ trials without the GO signal. We evaluated the consequences of disruption of activity of right and left M1, PMd and PMv cortices (one block each) on SRT. The order of trials in a block and the order of blocks were randomized within and across subjects. Up to two catch responses were accepted in each block. Catch trials, as administered in this study, ensured that subjects responded only to the GO signal (Pascual‐Leone et al., 1992a, b). Blocks with >two catch responses were not included in the final analysis and were repeated at the end of the session. SRT was defined as the time between the GO signal and the onset of the EMG burst (Fig. 2A). EMG was recorded from silver–silver chloride electrodes positioned in a belly‐tendon montage on the skin overlying the left and the right first dorsal interosseus (FDI) muscles. After amplification and bandpass filtering (50 Hz–2 kHz) (Counterpoint Electromygraph, Dantec Electronics, Skovlunde, Denmark), the EMG signal was digitized (sampling rate 5 kHz) and fed to a Macintosh computer for off‐line analysis of the waveforms. SRT onset was defined at the time the EMG amplitude exceeded 2SD of the baseline EMG.
TMS
Single‐pulse TMS was used to disrupt activity at each cortical site (Day et al., 1989; Desmurget et al., 1999; Pascual‐Leone et al., 1992a, b; Ziemann et al., 1997; Zangaladze et al., 1999) in an SRT paradigm (Day et al., 1989; Pascual‐Leone et al., 1992a, b; Ziemann et al., 1997; Johansen‐Berg et al., 2002). TMS pulses were delivered using figure 8 shaped magnetic coils of 7 and 5 cm diameter for real TMS and sham stimulation, respectively. TMS was delivered 120 ms after the GO signal to optimize SRT delays after stimulation of M1 in healthy volunteers (Day et al., 1989; Pascual‐Leone et al., 1992a, b; Ziemann et al., 1997). Magnetic coils were connected to two Magstim 200 magnetic stimulators (2.2 T; Magstim, Whitland, Dyfed, UK). TMS was delivered with the coil tangential to the scalp and perpendicular to the presumed direction of the central sulcus, 45° from the mid‐sagittal line, with the handle pointing backward (Mills et al., 1992). For sham stimulation, the coil was located in a similar position ∼5 cm away from the scalp. Resting motor threshold (RMT) was defined as the minimal stimulus intensity (percentage of the maximal stimulator output) that produced motor evoked potentials (MEPs) >50 µV peak‐to‐peak in amplitude in at least five out of 10 trials (Rossini et al., 1994). Two different stimulus intensities were used. In a first experiment, the intensity of TMS was 30% above RMT, determined separately in each hemisphere and individual. Sham stimulation was delivered at 30% above RMT plus an additional 5% of the maximum stimulator output to match the sound perception of the TMS pulse. Because RMTs in the affected hemisphere were higher than in the intact hemisphere or controls in two of the four individuals (see Results), we performed an additional control experiment. The affected hemisphere was stimulated with the same intensity as the intact hemisphere (30% above RMT of the intact M1).
Determination of target regions for TMS disruption
To target the same area in all of our subjects, we used a frameless, MRI‐guided stereotaxic system to guide the TMS coil position (Fig. 2B). Initially, we obtained T1‐weighted MRI in each subject using a 1.5 T scanner and a standard head coil, fast‐spoiled, gradient‐recalled at steady‐state images [TR (repetition time) = 11.2 ms; TE (echo time) = 2.1 ms; inversion time = 300 ms; flip angle = 30°, FOV (field of view) = 24 cm; 256 × 256 matrix; 124 slices; voxel size = 0.94 × 0.94 × 1.5 mm; GE, Milwaukee, WI, USA]. Subjects lay supine on a scanner bed. Foam cushions and elastic tape were used to minimize head motion. The MRI data were later fed to a Macintosh computer. Preceding the experimental session, anatomical locations of M1, PMd and PMv were identified in each MRI and projected in real‐time using Brainsight (Rogue Research, Montreal, Canada) over each subject’s scalp surface.
Anatomical landmarks
M1 was identified in axial [hand knob, seen in all subjects (Yousry et al., 1997)] and sagittal [hook, i.e. inconstantly seen at the plane of the insular cortex (Yousry et al., 1997)] views of the brain. Focal lesions in this anatomical location produce selective neurological deficits of the hand and wrist (Back and Mrowk, 2001; Gass et al., 2001; Takahashi et al., 2002). The projection of this location onto the scalp correlates closely with the location of the ‘hot spot’ in TMS studies in healthy volunteers (Gugino et al., 2001). This site—identified in the three‐dimensional view—matches activation sites in PET (Wassermann et al., 1996) and functional MRI studies (Boroojerdi et al., 1999). PMd stimulation targeted the posterior part of the middle frontal gyrus, consistent with previous TMS experiments (Schluter et al., 1998, 1999; Praamstra et al., 1999; Gerschlager et al., 2001; Munchau et al., 2002; Johansen‐Berg et al., 2002) and neuroimaging studies (Kertzman et al., 1997; Fink et al., 1997; Toni et al., 1999). The projection of this cortical site over the scalp rendered locations similar to those that, upon application of TMS, led to behavioural disruption consistent with PMd function in previous studies (Schluter et al., 1998, 1999; Praamstra et al., 1999; Johansen‐Berg et al., 2002). PMv stimulation targeted Brodmann area (BA) 44, located in the posterior part of the inferior frontal gyrus (Binkofski et al., 1999; Buccino et al., 2001).
Statistical analysis
The endpoint measure of the study was the TMS‐induced delay in SRT relative to sham in each block. Data were normally distributed (Kolmogorov–Smirnov test). Between groups, differences were analysed using unpaired 2‐way t statistics. Reaction times, MEP amplitudes and latencies, and MEP amplitude and latency ratios were analysed using a factorial ANOVA (analysis of variance) design with factors GROUP (healthy subjects/stroke patients), LOCATION (M1/PMd/PMv), and SIDE (intact, affected in patients). Comparisons between stroke and controls were performed for the same hemisphere. Post hoc pairwise comparisons were implemented using Scheffe’s test. Results were considered significant at a level of P = <0.05.
Results
All patients and controls completed the experimental protocol. RMTs (mean ± SE) were 49.8 ± 3.7% and 50.6 ± 3.9% for the right and left hemisphere of healthy controls, [not significant (ns)] and 48.2 ± 2.6% and 57 ± 3.6% for the intact and affected hemispheres of stroke patients (ns). In sham trials, SRT was faster in healthy controls than in the paretic hand of stroke patients (210.5 ± 3.8 ms and 245 ± 5 ms, respectively; P = 0.0001). ANOVA for movement times showed a significant effect of GROUP (F = 20.096, P = 0.002), but not LOCATION (F = 0.386, P = ns) nor their interaction (F = 0.426, P = ns).
Effects of TMS on SRTs
Overall, ANOVA demonstrated significant effects of factors GROUP (F = 6.9, P = <0.02), SIDE (F = 16.2, P = 0.0002), and LOCATION (F = 28.1, P = <0.0001), as well as GROUP × SIDE (F = 27, P = <0.0001), GROUP × LOCATION (F = 3.9, P = 0.02), SIDE × LOCATION (F = 7.5, P = 0.001), and GROUP × SIDE × LOCATION (F = 8.7, P = 0.0007) interactions on SRT (Figs 3 and 4). In healthy subjects, stimulation of M1 elicited substantial SRT delays in the contralateral hand (right 59.1 ± 13.5 ms, left 46.6 ± 6.8 ms; Fig. 4) that were longer than those elicited by stimulation of PMd (right 9.5 ± 5.8 ms, P = 0.007; left 2.9 ± 4.7 ms, P = 0.0004) and PMv (right 1.2 ± 5.1 ms, P = 0.002; left 1.2 ± 4.2 ms, P = 0.0002). There were no significant differences between SRT delays elicited by PMd and PMv stimulation (Fig. 4).
In stroke patients, stimulation of the intact hemisphere revealed differences similar to those reported in the control group. Stimulation of the M1 intact hemisphere (M1IH) elicited marked delays in contralateral SRT (30.8 ± 7.3 ms) that were longer than those produced by stimulation of the PMd intact hemisphere (PMdIH) (–11.3 ± 7.4 ms, P = 0.01) or the PMv intact hemisphere (PMvIH) (3.0 ± 8.7 ms, P = 0.09; Fig. 4). Similar to the findings in controls, there were no significant differences between SRT delays elicited by PMdIH and PMvIH stimulation.
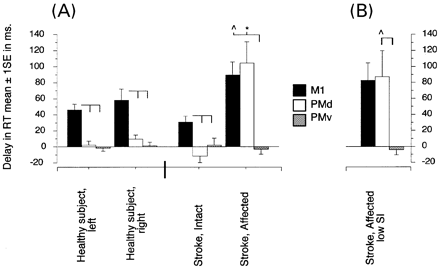
Evaluation of the affected hemispheres of patients revealed a different pattern. First, at an intensity of 130% MT of the affected hemisphere (Fig. 4A), stimulation of the M1 affected hemisphere (M1AH) and PMd affected hemisphere (PMdAH) elicited similar delays in SRT of the paretic hand (90.3 ± 16 ms and 104.6 ± 26.9 ms, respectively) that were longer than with stimulation of the PMv affected hemisphere (PMvAH) (–2.0 ± 6.8 ms, P < 0.02 and P < 0.009, respectively; Fig. 4A). Moreover, stimulation of PMdAH caused longer SRT delays in the paretic hand than stimulation of PMdIH in the intact hand (P < 0.006) of patients and healthy controls (P < 0.004). The SRT delay elicited by stimulation of M1AH was longer than delays identified by stimulation of the same region in healthy controls (P < 0.03) and the M1IH of patients (P < 0.01). SRT delays elicited by stimulation of the intact hemisphere of patients and any hemisphere of healthy controls were similar.
As stated above, MT in the affected hemisphere was higher than in the intact hemisphere in two of the patients. To control for this difference, we adjusted the intensity of TMS in the affected hemisphere to match the lower intensity used in the intact hemisphere (130% MT of the intact hemisphere). The results were similar to those reported above (see Fig. 4B). Stimulation of M1AH (73.9 ± 21.5 ms) and PMdAH (78.3 ± 26.2 ms) elicited similar SRT delays in the contralateral paretic hand (Fig. 4B). SRT delay after PMdAH stimulation was significantly longer than after stimulation of PMdIH (P < 0.02).
MEPs
Amplitudes
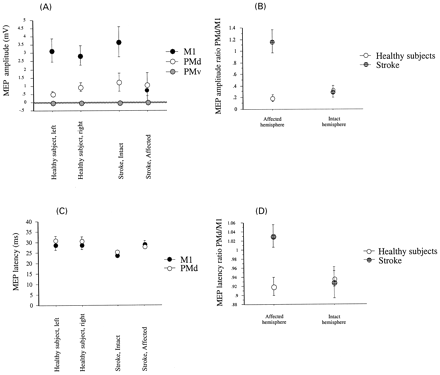
Overall, ANOVA demonstrated significant effects of SIDE (F = 4, P < 0.05) and LOCATION (F = 33.1, P < 0.0001), as well as GROUP × SIDE × LOCATION (F = 4.5, P = 0.01) interactions on MEP amplitudes (Figs 3 and 5). In healthy subjects, stimulation of M1 elicited larger MEPs (right 2.8 ± 0.5 mV, left 3.1 ± 0.6 mV; Fig. 5A) than stimulation of PMd (right 0.9 ± 0.2 mV, P = 0.002; left 0.5 ± 0.1 mV, P = 0.01). PMv stimulation did not elicit contralateral MEP in any subject. In stroke patients, stimulation of M1IH elicited results similar to those observed in healthy controls: larger MEP amplitudes in the contralateral intact hand (3.7 ± 0.9 mV; Fig. 5A) than stimulation of PMdIH (1.2 ± 0.5 mV, P = 0.0501). PMvIH stimulation did not elicit contralateral MEPs in any patient.
In contrast, stimulation of M1AH and PMdAH elicited MEPs of similar amplitudes (0.7 ± 0.3 mV and 1 ± 0.7mV respectively, P = ns; Fig. 5A). Between‐group comparison showed that contralateral MEPs elicited by stimulation of M1AH were smaller than those elicited by stimulation of homologous areas in healthy subjects and patients (P = 0.02). Consequently, PMd/M1 MEP amplitude ratio was greater in the affected hemisphere of patients than in controls and than in the intact hemisphere of stroke patients (P < 0.001 and P < 0.008, respectively; Fig. 5B).
Latencies
ANOVA demonstrated significant effects of GROUP (F = 6.2, P = 0.01; Fig. 5C) on contralateral MEP latencies, reflecting a non‐significant trend for shorter latencies with M1 than with PMd stimulation, particularly after stimulation of the intact hemispheres of controls and patients. More important, MEP latencies were, on average, shorter with stimulation of PMdAH than with stimulation of M1AH (Fig. 5C). Consequently, the PMd/M1 MEP latency ratio was greater in the affected hemisphere of patients than in controls and than in the intact hemisphere of the patient group (P < 0.01; Fig. 5D). Ipsilateral MEPs from both FDI were identified in one of two subjects tested.
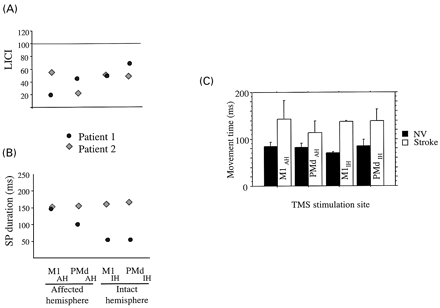
Long‐latency intracortical inhibition (LICI) and silent periods (SP)
We tested LICI to stimulation of M1 and PMd in two of the subjects who experienced clear delays in RT after PMdAH relative to stimulation of the control positions. Two TMS stimuli 100 ms apart were given at 120% of resting motor threshold (Chen et al., 1999; Daskalakis et al., 2002; Di Lazzaro et al., 2002). We did not find consistent changes in LICI after PMdAH stimulation in these two patients (Fig. 6A). Additionally, we evaluated SP applying TMS pulses at 140% MT during low‐force activation of FDI (15–20% of maximal force) to M1 and PMd of both hemispheres (Fuhr et al., 1991; Wassermann et al., 1993; Ziemann et al., 1993). The SP was measured from the end of the MEP to the start of subsequent EMG activity. The main result of this experiment was comparable SP in both hemispheres of one of the patients and longer SP in the affected hemisphere of the other patient (Fig. 6B). We found no correlation between LICI and SP and RT delays elicited by stimulation at any of the four stimulation sites.
Discussion
The main finding of this study is that TMS applied to the PMd of the affected hemisphere in a group of chronic stroke patients with selective lesions of CS originating in M1 and satisfactory motor recovery disrupted motor behaviour in the paretic hand.
Role of non‐primary motor regions in recovery of motor function after stroke
The substrates underlying recovery of motor function after chronic stroke are incompletely understood. However, recent investigations have started to shed light on the contribution of non‐primary motor areas. In humans, the involvement of the PMd of the intact hemisphere in recovery of motor function in chronic stroke patients has been documented particularly in those with more severe disability (Johansen‐Berg et al., 2002; Ward et al., 2003). The substrates underlying recovery in cases where motor recovery is more substantial are not known. A possible clue arose from recent primate studies that proposed that functional recovery after focal lesions in M1 may be mediated by reorganized activity in non‐primary motor regions of the lesioned hemisphere, such as the PMd and PMv (Liu and Rouiller, 1999; Frost et al., 2003). This proposal was consistent with human studies that identified increased activation in the PMd of the lesioned hemisphere in patients with chronic stroke who experienced substantial motor recovery (Weiller et al., 1992, 1993; Seitz et al., 1998; Mima et al., 2001; Carey et al., 2002) and with TMS studies that reported an anterior displacement of the centre of gravity of TMS maps obtained after stimulation of the affected hemisphere of chronic stroke patients who recovered motor function (Liepert et al., 1998; Byrnes et al., 1999, 2001). Overall, these considerations led us to investigate the untested hypothesis of functional contribution of PMd of the lesioned hemisphere in chronic stroke patients with good motor recovery and homogeneous and focal subcortical lesions.
We chose a group of patients with comparable lesion size, lesion location and degree of motor recovery. The four patients tested in our experiment satisfied strict inclusion criteria: they had focal lesions located in the posterior two‐thirds of the posterior limb of the internal capsule (PLIC), a region that conducts corticofugal fibres originating in M1 but not those originating in PMd or PMv (Fig. 1; Fries et al., 1993; Morecraft et al., 2002). The finding of smaller MEP amplitudes in the paretic hand after stimulation of M1AH relative to stimulation of control hemispheres and the similarity of MEP amplitudes elicited after stimulation of PMd in the affected and control hemispheres are consistent with the view that the anatomical location of the lesions disrupted preferentially corticospinal outflow originating in M1, but preserved the pathways mediating the response to stimulation of PMd (see Fig. 5B). All patients experienced good recovery of motor function according to a standardized impairment scale (MRC). We chose these stringent inclusion criteria to reduce the possibility that various lesion sites, locations and degrees of disability could confound evaluation of the primary hypothesis under investigation.
The identification of stimulation sites was performed on an anatomical MRI basis to avoid the bias generated by the predicted anterior migration of functional landmarks, such as the ‘hot spot’ (functional measure to localize M1 when using TMS) in the affected hemisphere (Liepert et al., 1998; Byrnes et al., 1999, 2001). Therefore, we localized M1, PMd and PMv in the MRIs of each patient and healthy volunteer, and projected those locations onto the scalp using a frameless stereotaxic technique successfully used in previous investigations (Boroojerdi et al., 1999; Gugino et al., 2001). This approach allowed us to test the functional role of each specific brain region in the absence of biasing assumptions. The anatomical regions chosen were consistent with those identified in previous TMS, functional neuroimaging and lesion studies in humans: the hand knob (M1) (Yousry et al., 1997; Gass et al., 2001; Back and Mrowka, 2001), the most posterior part of the middle frontal gyrus that corresponds to the most anterior part of PMd (Kertzman et al., 1997; Schluter et al., 1998, 1999; Toni et al., 1999; Gerschlager et al., 2001; Munchau et al., 2002), and the most posterior part of the inferior frontal gyrus (PMv or BA 44) (Binkofski et al., 1999; Buccino et al., 2001; Rizzolatti et al., 2002). PMd stimulation was applied over the most posterior part of the middle frontal gyrus to minimize spread to M1.
The behavioural paradigm was the SRT, in which subjects perform a stereotyped, preprogrammed, ballistic voluntary movement in response to a GO signal. In healthy volunteers, we found that stimulation of M1, but not PMd, elicited clear delays in contralateral reaction times—a result consistent with previous reports (Day et al., 1989; Pascual‐Leone et al., 1992a, b; Ziemann et al., 1997). This delay has been interpreted as indicative of the involvement of M1 in motor execution (Day et al., 1989; Pascual‐Leone et al., 1992b). Stimulation of the intact hemisphere of stroke patients revealed results similar to those found in healthy controls. TMS applied to M1IH elicited delays in contralateral SRT in the patients’ intact hands in the absence of changes with PMdIH or PMvIH stimulation. The delay in SRT in the intact hand during disruption of M1IH was less impressive than in healthy subjects, possibly because of the relatively longer absolute SRT in the intact hand of patients compared with healthy controls (Pascual‐Leone et al., 1992a; Ziemann et al., 1997). Stimulation of M1AH of stroke patients resulted in delays in contralateral SRT in the paretic hand that were longer than with stimulation of control hemispheres (Fig. 4); this is consistent with previous reports in humans (Werhahn et al., 2003; Murase et al., 2004) and with primate studies (Nudo and Milliken, 1996; Nudo et al., 1996; Rouiller et al., 1998). All together these findings are consistent with the view that reorganized regions of M1AH participate as an active substrate that contributes to functional recovery.
The fundamental novel finding in this study is that stimulation of PMd of the affected hemisphere, PMdAH, elicited a marked delay in contralateral SRT in the paretic hand that was not evidenced by stimulation of the same region in control hemispheres. This delay was also found when lower TMS intensities were used in the two patients with higher motor thresholds (see Fig. 4B). Therefore, differences in TMS intensities across hemispheres could not account for our findings. In addition to SRT delays, stimulation of PMdAH of patients elicited contralateral MEPs in the paretic hand. The mean amplitude of MEP elicited by PMdAH stimulation was slightly larger than the amplitude of MEP elicited by M1AH stimulation (Fig. 5A and B); this is consistent with the view that the PMd of the lesioned hemisphere has physiologically active corticomotoneuronal connections at least as strong (if not stronger in these particular patients) as those that remain intact in M1AH. The finding of slightly shorter MEP latencies after stimulation of PMdAH relative to stimulation of M1AH—a situation reversed in control hemispheres (see Fig. 5C and D)—further supports this contention and is consistent with the interpretation that PMdAH contributes to recovery of motor function of the paretic hand in this patient group. Further support for this interpretation comes from: (i) the finding that spontaneous partial recovery from weakness following a focal experimental lesion in M1 is inhibited by muscimol injections of PMd in the lesioned hemisphere, but not by muscimol injection of PMd in the intact hemisphere (Liu and Rouiller 1999); and (ii) activation of ipsilesional PMd in association with stroke recovery in human functional neuroimaging studies (Weiller et al., 1992, 1993; Seitz et al., 1998; Carey et al., 2002). In summary, these findings identify, for the first time to our knowledge, a functionally relevant involvement of ipsilesional PMd in functional recovery after human stroke.
Mechanisms underlying delays in SRT in the paretic hand by stimulation of ipsilesional PMd
Although the mechanisms underlying this effect are currently unknown, several possibilities can be advanced. First, it is possible that PMd stimulation in our study and muscimol injections in the study of Liu and Rouiller (1999) disrupted the activity of movement‐related neurons in the caudal aspect of PMd (PMdc) (Weinrich and Wise, 1982; Weinrich et al., 1984; Kurata and Wise, 1988; Boussaoud and Wise, 1993), a region with pyramidal cells in layer V that have direct projections to the spinal cord (Murray and Coulter, 1981; Dum and Strick, 1991, 1996; He et al., 1993). It is conceivable that these connections (physiologically less active in the normal brain) became unmasked (Lee and van Donkelaar, 1995), perhaps as a result of Hebbian learning (Pennartz, 1997) after the stroke leading to short latency MEP from PMdAH. Secondly, it is theoretically possible that the excitability of intracortical inhibitory circuits within M1AH was abnormally high (Classen, 1997; Traversa, 2000). Techniques like suprathreshold paired pulse intracortical inhibition (ICI) and a detailed topographic analysis of SP correlated with RT delays in a larger population of stroke patients could provide additional information on this issue (Ziemann, 1997). Thirdly, the observed SRT delays could be caused by disruption of reorganized activity in rostral PMdAH. However, this region, albeit close to the stimulating TMS coil, is active in processing higher aspects of sensorimotor integration (Picard and Strick, 2001) and non‐motor mental‐operation tasks (numeric, verbal and spatial) (Takahashi et al., 2002), but lacks direct corticospinal projections (Picard and Strick, 2001). It is possible that spread from PMd to M1 could cause these results. First, PMd stimulation led to SRT delay only after stimulation of the affected hemisphere of patients. Secondly, stimulation of PMdIH elicited MEP latencies longer than stimulation of M1IH, but stimulation of PMdAH elicited, on average, MEP latencies shorter than stimulation of M1AH (see Fig. 5). Finally, the finding of stable MEP amplitudes to PMdAH stimulation in the presence of small MEP to M1AH stimulation is inconsistent with spread (see Fig. 5). One additional consideration is that the moderately longer RT in patients resulted in a relatively different timing of TMS application in relation to movement onset, possibly towards a more vulnerable premovement period in the patient group.
TMS over PMvAH failed to elicit SRT delays in the paretic hand. The PMv in non‐human primates is divided into two subregions, F5, or rostral PMv, and F4, or caudal PMv (Rizzolatti et al., 2002). Both of these regions experience cortical reorganization after M1 lesions (Liu and Roullier, 1999; Frost et al., 2003; see Fig. 1). The monkey F4, a region with giant pyramidal cells in layer V and direct projections to the spinal cord (Dum and Strick, 1991; He et al., 1993; Picard and Strick, 2002), is thought to be involved in the transformation of a target objective into a movement goal in an intrinsic reference frame (Kakei et al., 2001). However, the homologue of F4 has not been definitively identified in humans (Rizzolatti et al., 2002). On the other hand, F5 (and its putative human homologue B44) lacks direct corticospinal connections and pyramidal cells in layer V (Dum and Strick, 1991, 1996; He et al., 1993) and is involved in recognition of motor actions (Umilta et al., 2001), as well as in imitation (Iacoboni et al., 1999) and visuomotor (Fogassi et al., 2001) and sensorimotor (Binkofski et al., 1999) transformations for grasping and manipulation. Because our target for TMS was B44, it is not surprising that TMS over this site failed to disrupt SRT of finger movements. More detailed PMv mapping experiments, as well as more specific tasks, will be required to evaluate the role of this region in recovery of motor function after human stroke.
In summary, the findings described here, together with those of recent reports (Johansen‐Berg et al., 2002), are consistent with an active role of the PMd in stroke recovery that is influenced by lesion sites and degree of recovery. The PMd in the affected hemisphere may contribute to functional recovery when lesions are focal, involving M1 or CS originating in M1 (Liu and Roullier 1999; Frost et al., 2003; our results), in patients with better recovery. The PMd in the intact hemisphere may play a role with larger lesions in more extensive middle cerebral artery territories leading to more prominent disability (Johansen‐Berg et al., 2002; Ward et al., 2003).
Acknowledgements
The authors wish to thank S. P. Wise for comments on a previous version of this paper. Partial information from this paper was presented in April 2002 during the 3rd World Conference of Neurological Rehabilitation (Venice, Italy) and at the 2002 meeting of the American Academy of Neurology (Denver, Colorado, USA).
Fig. 1 T1 MRIs from the four patients studied. The arrows indicate the lesion sites. The diagrams at the centre show the location of the pyramidal tract fibres originating in M1 in the primate brain according to Morecraft et al. (2002). See Fries et al. (1993) for similar information in humans.
Fig. 2 (A) Experimental design: the warning signal is followed at random intervals of 1–3 s by a GO signal commanding index finger flexion. TMS was applied 120 ms following the GO signal. (B) Anatomical locations projected onto the scalp (see Methods). CS = central sulcus.
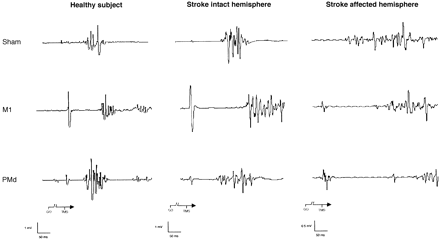
Fig. 3 Representative EMG recordings from contralateral FDI muscle with sham, M1 and PMd stimulation in a healthy volunteer and in Patient 1. Note that contralateral SRTs were delayed by stimulation of M1 in the healthy volunteer and by stimulation of M1IH and M1AH of the patient relative to sham. Stimulation of PMdAH produced a marked delay in contralateral SRT in the affected hand of the stroke patient, but did not affect contralateral SRT after stimulation of any PMd in a healthy volunteer nor after stimulation of PMdIH in the patient.
Fig. 4 (A) Group data showing contralateral SRT delays with TMS of different cortical sites. Note that M1 stimulation (black bars) applied to healthy volunteers and to either hemisphere of patients elicited significant contralateral SRT delays. PMd stimulation (open bars) elicited significant contralateral SRT delays when applied to the affected hemisphere of patients. In contrast, PMd stimulation applied to healthy volunteers and to the intact hemisphere of the patient group did not affect SRT. PMv stimulation did not elicit SRT delays. (B) Application of TMS to the PMd of the affected hemisphere at 130% of MT of the intact motor cortex (at lower intensity than in (A) led to the same result. Data expressed as mean ± SE. *P = <0.05 (within group); ∧P = <0.05 (between groups).
Fig. 5 Group data showing MEP amplitudes (A) and latencies (C) and MEP amplitude ratio (B) and latency ratios (D) of PMd stimulation/M1 stimulation in the different groups. (A) M1 stimulation applied to healthy subjects and to the intact hemisphere of patients elicited clear MEP responses of ∼3 mV. Stimulation of M1 in the affected hemisphere of patients elicited lesser responses (<1 mV). PMd stimulation applied to healthy subjects and to the intact hemisphere of patients elicited relatively small MEPs (0.5–1 mV). Stimulation of the PMd of the affected hemisphere of patients elicited MEPs of amplitude similar to controls (close to 1 mV). Stimulation of PMv did not elicit MEP. Note that stimulation of M1 of the affected hemisphere elicited smaller MEPs than stimulation of other locations, while stimulation of the PMd of the affected hemisphere elicited MEPs of amplitude similar to those elicited by stimulation of other sites. (B) PMd/M1 MEP amplitude ratio was very small in both hemispheres of healthy volunteers and in the intact hemisphere of patients, reflecting very small responses to PMd stimulation. On the other hand, the same ratio was significantly higher in the affected hemisphere of patients, most likely reflecting preserved MEP amplitudes after stimulation of PMd in the affected hemisphere. (C) Although MEP latencies elicited after stimulation of M1 and PMd were similar overall, stimulation of the PMd in the affected hemisphere elicited mean latencies slightly shorter than those elicited by stimulation of M1 of the affected hemisphere. For comparison, note that mean MEP latencies elicited by stimulation of PMd in control hemispheres were longer than those elicited by stimulation of M1. (D) PMd/M1 MEP latency ratio was very small in healthy volunteers and in the intact hemisphere of patients. However, the same ratio was significantly higher in the affected hemisphere of patients, most likely reflecting the reversal of latency ratios described in C.
Fig. 6 LICI (A) and SP (B) in two of the patients (see Results for description). Movement times (C) in normal volunteers and patients with stimulation at the different sites. Overall ANOVA was performed with factors Group (stroke patients, NV) and Location (four sites) on movement times. Results indicated significant effects of Group (F = 20.096, P = 0.002), but not Location (F = 0.386, P = ns) nor interaction Group × Location (F = 0.426, P = ns), pointing to longer movement times in stroke patients than controls in the absence of differential effects with TMS.
Patient characteristics
| Patient | Age (years) | Sex | Handed | Time after stroke (years) | Initial grade of paresis | MRC (hand) | Neurological deficit |
| 1 | 73 | Female | Right | 11 | Severe | 3+ | Pure motor |
| 2 | 81 | Male | Right | 7 | Severe | 4+ | Pure motor |
| 3 | 73 | Female | Right | 2 | Moderate to severe | 4 | Pure motor |
| 4 | 77 | Male | Right | 3.5 | Severe | 4 | Pure motor |
| Patient | Age (years) | Sex | Handed | Time after stroke (years) | Initial grade of paresis | MRC (hand) | Neurological deficit |
| 1 | 73 | Female | Right | 11 | Severe | 3+ | Pure motor |
| 2 | 81 | Male | Right | 7 | Severe | 4+ | Pure motor |
| 3 | 73 | Female | Right | 2 | Moderate to severe | 4 | Pure motor |
| 4 | 77 | Male | Right | 3.5 | Severe | 4 | Pure motor |
Patient characteristics
| Patient | Age (years) | Sex | Handed | Time after stroke (years) | Initial grade of paresis | MRC (hand) | Neurological deficit |
| 1 | 73 | Female | Right | 11 | Severe | 3+ | Pure motor |
| 2 | 81 | Male | Right | 7 | Severe | 4+ | Pure motor |
| 3 | 73 | Female | Right | 2 | Moderate to severe | 4 | Pure motor |
| 4 | 77 | Male | Right | 3.5 | Severe | 4 | Pure motor |
| Patient | Age (years) | Sex | Handed | Time after stroke (years) | Initial grade of paresis | MRC (hand) | Neurological deficit |
| 1 | 73 | Female | Right | 11 | Severe | 3+ | Pure motor |
| 2 | 81 | Male | Right | 7 | Severe | 4+ | Pure motor |
| 3 | 73 | Female | Right | 2 | Moderate to severe | 4 | Pure motor |
| 4 | 77 | Male | Right | 3.5 | Severe | 4 | Pure motor |
References
Binkofski F, Buccino G, Posse S, Seitz R, Rizzolatti G, Freund J. A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study.
Boroojerdi B, Foltys H, Krings T, Spetzger U, Thron A, Topper R. Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging.
Boussaoud D, Wise SP. Primate frontal cortex: effects of stimulus and movement.
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study.
Butefisch C, Hummelsheim H, Denzler P, Mauritz K. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand.
Byrnes ML, Thickbroom GW, Phillips BA, Wilson SA, Mastaglia FL. Physiological studies of the corticomotor projection to the hand after subcortical stroke.
Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long‐term changes in motor cortical organisation after recovery from subcortical stroke.
Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke.
Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings.
Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, et al. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic stroke.
Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L. Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex.
Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, et al. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain.
Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: a positron emission tomography study.
Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long‐term survival after first‐ever stroke: the Oxfordshire Community Stroke Project.
Denny‐Brown D, Botterell EH. The motor function of the agranular frontal cortex.
Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target.
Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, et al. Direct demonstration of long latency cortico‐cortical inhibition in normal subjects and in a patient with vascular parkinsonism.
Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe.
Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys.
Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements.
Ferrucci L, Bandinelli S, Guralnik JM, Lamponi M, Bertini C, Falchini M, Baroni A. Recovery of functional status after stroke: a post rehabilitation follow‐up study.
Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex.
Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey. A reversible inactivation study.
Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke: role of descending pathways from multiple motor areas.
Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery.
Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation.
Gass A, Szabo K, Behrens S, Rossmanith C, Hennerici M. A diffusion‐weighted MRI study of acute ischemic distal arm paresis.
Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex.
Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual‐Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials.
Halsband U, Freund HJ. Premotor cortex and conditional motor learning in man.
Halsband U, Ito N, Tanji J, Freund HJ. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man.
He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere.
Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta J, Rizzolatti G. Cortical mechanisms of human imitation.
Johansen‐Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke.
Kakei S, Hoffman DS, Strick PL. Direction of action is represented in the ventral premotor cortex.
Kelly‐Hayes M, Wolf P, Kase C, Gresham G, Kanne W, D’Agostino R. Time course of functional recovery after stroke.
Kertzman C, Schwarz U, Zeffiro TA, Hallett M. The role of posterior parietal cortex in visually guided reaching movements in humans.
Kotila M, Waltimo O, Niemi M, Laaksonen R, Lempinen M. The profile of recovery from stroke and factors influencing outcome.
Kurata K, Wise SP. Premotor cortex of rhesus monkeys: set‐related activity during two conditional motor tasks.
Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. [Review].
Liepert J, Miltner W, Bauder H, Sommer B, Dettmers C, Taub E, et al. Motor cortex plasticity during constraint‐induced movement therapy in stroke patients.
Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys.
Medical Research Council. Aids to the examination of the peripheral nervous system. London: HMSO;
Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation.
Mima T, Toma K, Koshy B, Hallett M. Coherence between cortical and muscular activities after subcortical stroke.
Morecraft RJ, Herrick JL, Stilwell‐Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, et al. Localization of arm representation in the corona radiata and internal capsule in the non‐human primate.
Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation.
Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic subcortical stroke.
Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey.
Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys.
Nudo RJ, Wise BM, Sifuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory.
Pascual‐Leone A, Brasil‐Neto J, Valls‐Sollé J, Cohen L, Hallett M. Simple reaction time to focal transcranial magnetic stimulation. Comparison with reaction time to acoustic, visual and somatosensory stimuli.
Pascual‐Leone A, Valls‐Sole J, Wassermann EM, Brasil‐Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli.
Pennartz CM. Reinforcement learning by Hebbian synapses with adaptive thresholds.
Praamstra P, Kleine BU, Schnitzler A. Magnetic stimulation of the dorsal premotor cortex modulates the Simon effect.
Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex.
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. [Review].
Rossini PM, Calautti C, Pauri F, Baron JC. Post‐stroke plastic reorganisation in the adult brain.
Rouiller EM, Yu XH, Moret V, Tempini A, Wiesendanger M, Liang F. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion.
Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation.
Schluter ND, Rushworth MF, Mills KR, Passingham RE. Signal‐, set‐, and movement‐related activity in the human premotor cortex.
Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions.
Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction.
Takahashi N, Kawamura M, Araki S. Isolated hand palsy due to cortical infarction: localization of the motor hand area.
Toni I, Schluter ND, Josephs O, Friston K, Passingham RE. Signal‐, set‐ and movement‐related activity in the human brain: an event‐related fMRI study.
Traversa R, Cicinelli P, Oliveri M, Palmieri MG, Filippi MM, Pasqualetti P, et al. Neurophysiological follow‐up of motor cortical output in stroke patients.
Travis AM. Neurological deficiencies after ablation of the precentral motor area in Macaca mulatta.
Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, et al. I know what you are doing: a neurophysiological study.
Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: a cross‐sectional fMRI study.
Wassermann E, Pascual‐Leone A, Valls‐Sole J, Toro C, Cohen LG, Hallett M. Topography of the inhibitory and excitatory responses to transcranial magnetic stimulation in a hand muscle.
Wassermann EM, Wang B, Zeffiro TA, Sadato N, Pascual‐Leone A, Toro C, et al. Locating the motor cortex on the MRI with transcranial magnetic stimulation and PET.
Weiller C, Chollet F, Friston KJ, Wise RJS, Frackowiak RSJ. Functional reorganization of the brain in recovery from striatocapsular infarction in man.
Weiller C, Ramsay S, Wise RP, Friston KJ, Frackowiak RSJ. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction.
Weinrich M, Wise SP, Mauritz KH. A neurophysiological study of the premotor cortex in the rhesus monkey.
Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke.
Wise S. Evolution of neuronal activity during conditional motor learning. In: Bloedel JR, Ebner TJ, Wise SP, editors. The acquisition of motor behaviour in vertebrates. Cambridge (MA): MIT Press;
Wu CP, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates.
Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark.
Zangaladze A, Epstein CM, Grafton ST, Sathian K. Involvement of visual cortex in tactile discrimination of orientation.
Ziemann U, Netz J, Szelenyi A, Hömberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex.