-
PDF
- Split View
-
Views
-
Cite
Cite
Amy Christensen, Paul Micevych, CAV1 siRNA Reduces Membrane Estrogen Receptor-α Levels and Attenuates Sexual Receptivity, Endocrinology, Volume 153, Issue 8, 1 August 2012, Pages 3872–3877, https://doi.org/10.1210/en.2012-1312
Close - Share Icon Share
Although classic estrogen receptors (ER) have been proposed to mediate estradiol signaling, it has been relatively recently that mechanisms of trafficking these receptors have been elucidated. ERα is palmitoylated and associates with caveolin proteins to be targeted to the cell membrane. Caveolins are scaffold proteins that not only traffic ERα to the membrane but also are involved in establishing metabotropic glutamate receptor interactions that are necessary for activating G protein signaling. To demonstrate the role of caveolin proteins in regulating an estradiol-dependent behavior, sexual receptivity, we used small interfering RNA to knock down caveolin-1 (CAV1) expression in the arcuate nucleus of the hypothalamus. In CAV1 knockdown rats, membrane, but not intracellular levels of ERα, were significantly reduced. As expected, estrogenic stimulation of the arcuate nucleus of the hypothalamus to medial preoptic nucleus projection was abrogated in CAV1 knockdown rats, indicating that the membrane-initiated activation of this circuit was compromised. Moreover, estradiol-induced lordosis behavior that is dependent on activation of μ-opioid receptors in the medial preoptic nucleus was also significantly reduced. Thus, CAV1-mediated ERα trafficking to the cell membrane is required for estradiol activation of circuits underlying female sexual receptivity.
Cell membranes have been demonstrated to have specialized areas, referred to as caveolae, in which cell signaling machinery is clustered (1, 2). Neurons do not have caveolae-like invaginations, but their membranes are rich in caveolin proteins that define functional microdomains of the cellular membrane. Such lipid rafts allow for the segregation of signaling pathways (3). Caveolins, a family of scaffold proteins, participate in targeting receptors and associated proteins to the membrane (4). Estrogen receptor (ER)-α has been demonstrated to interact with caveolin proteins, which are involved in trafficking of this receptor to the cell membrane and coupling it with its signaling partners (5, 6).
In vitro, ERα has been shown to interact with different metabotropic glutamate receptors (mGluR) through specific caveolin proteins (6). Caveolin-1 (CAV1) mediates the ERα interaction with type 1 mGluR, mGluR1 and mGluR5, whereas type 2 mGluR, mGluR2 and mGluR3, are associated with caveolin-3 (reviewed in Ref. 7). The specificity of these interactions promotes different types of signaling. ERα transactivation of mGluR1 stimulates phospholipase C-dependent pathways leading to activation of the MAPK pathway that phosphorylates cAMP response element binding protein and releases internal calcium stores (6, 8). However, when estradiol-activated ERα interacts with type 2 mGluR, adenylyl cyclase is blocked and flux through the L-type calcium channel is reduced (9, 10). In this way both the facilitation and inhibition mediated by ERα is explained.
ERα is an important component of the induction of sexual receptivity by estradiol (11, 12). Estradiol has been shown to act through membrane receptors in the arcuate nucleus of the hypothalamus (ARH) to rapidly activate a circuit that extends from the ARH to medial preoptic nucleus (MPN) to ventromedial hypothalamic nucleus, mediating lordosis behavior (reviewed in Ref. 13). A hallmark of estrogen membrane signaling is ERα transactivation of mGluR in the ARH [(14–16) and reviewed in Ref. 7)], leading to the release of β-endorphin (β-END) and the internalization of μ-opioid receptor (MOR) in the MPN (17).
In vitro, levels of ERα and mGluR1 on the membrane are regulated by estradiol treatment through activation of the ER-mGluR1a complex (18, 19). Estradiol transiently increases both receptors on the membrane at a time point that coincides with increased receptor activation and downstream signaling. To determine whether there is a physiological role for caveolin-mediated ERα trafficking to the membrane in the ARH, we used a site-specific knockdown of CAV1, the most likely caveolin protein mediating the association with mGluR1a, an interaction that is important for estradiol stimulation of neuroprogesterone synthesis and lordosis behavior (15, 20). Small interfering RNA (siRNA) treatment reduced CAV1 expression and prevented the trafficking of ERα to the cell membrane. Estradiol activation/internalization of MOR was abrogated in rats with CAV1 knocked down in the ARH, and the lordosis quotient, a measure of sexual receptivity, was significantly reduced.
Materials and Methods
Animals
Male and ovariectomized (ovx; by the supplier) female (200–250 g) Long-Evans rats were purchased from Charles River (Portage, MI). Upon arrival, rats were housed two per cage in a climate-controlled room, with a 12-h light, 12-h dark cycle (lights on at 0600 h). Food and water were available ad libitum. All experimental procedures were approved by the Chancellor's Animal Research Committee at the University of California, Los Angeles (Los Angeles, CA).
Steroid priming
Animals were allowed to survive 2–3 wk after ovx before steroid treatment. For all experiments, 17β-estradiol benzoate (EB) dissolved in safflower oil was injected sc in a volume of 0.1 ml per rat. Females received 5 μg EB every 4 d between 0800 and 0900 h for three cycles to mimic the natural estrous cycle of female rats (21).
Guide cannulae implantation surgery
Bilateral guide cannulae (22 gauge; Plastics One Inc., Roanoke, VA) directed at the ARH (coordinates from bregma: anterior −2.8 mm, lateral 0.8 mm, ventral −7.4 mm from dura; tooth bar: −3.3 mm) were implanted using standard stereotaxic procedures while female rats were anesthetized with isoflurane (2–3% in equal parts oxygen and nitrous oxide). Cannulae were secured to the skull with dental acrylic and stainless steel bone screws. Stylets were placed in the guide cannulae, which protruded less than 0.5 mm beyond the opening of the guide cannulae. Animals were individually housed after surgery, received oral antibiotics (trimethoprim and sulfamethoxazole, 0.4 mg/ml; Hi-Tech Pharmacal, Amityville, NY) in the drinking water, and were allowed to recover 6–7 d before infusion.
Microinjection
For ARH site-specific microinjections, 2 μg/μl of CAV1 siRNA or control siRNA (ON-TARGETplus SMARTpool siRNA; Dharmacon, Lafayette, CO) were dissolved in artificial cerebrospinal fluid. One microliter per side of the siRNA was microinjected with an infusion pump (Harvard Apparatus, Holliston, MA) at a rate of 0.25 μl/min. Microinjection needles (28 gauge) protruded 1 mm or less beyond the opening of the cannula and were allowed to remain in place for 1 min after infusion to allow for diffusion away from the injector. After microinjection, the obturators were reinserted into the guide cannulae and animals were returned to their home cage.
To ensure a significant knockdown of CAV1, animals received four microinfusions, one each day: the first was 30 min before the second EB injection; and the second was the next day. The third microinfusion was 3 d after the EB injection, which was the day before the final EB injection. The final microinfusion was 30 min before the third, and last, EB injection.
Behavioral testing
Sexually experienced males were acclimated to the Plexiglas testing arenas for 15 min before testing. Thirty hours after the third EB injection, female sexual receptivity was measured by placing each female rat into an arena with a stud male. Stimulus males were allowed to mount females 10 times and the number of times the female displayed lordosis (lifting of the head, arching of the back, movement of the tail to one side) was recorded. For each female, the sexual receptivity was quantified as a lordosis quotient (LQ), the number of lordosis displays/number of mounts × 100 (15).
Confirmation of guide cannulae placement
Animals used for immunohistochemical studies only were anesthetized and transcardially perfused with chilled 0.9% saline, followed with a fixative, 4% paraformaldehyde dissolved in 0.2 m Sorenson's phosphate buffer (pH 7.4). Brains were removed and placed in fixative overnight at 4 C and then replaced with 20% sucrose in phosphate buffer for cryoprotection. Brains were blocked, sectioned (20 μm) on a cryostat (Leica CM 1800; Leica, Bannockburn, IL), and collected into chambers filled with PBS. Sections were mounted onto SuperFrost/Plus slides (Fisher, Pittsburgh, PA), stained with thionin, dehydrated, and coverslipped with Krystalon (EMD Chemicals, Gibbstown, NJ). Injection sites were mapped and verified with bright-field illumination. In animals used for Western blot analysis, cannula position was confirmed visually during dissection.
Rats with cannulae that were not positioned in the ARH (i.e. located above, lateral to the ARH, or where microinjections had compromised the wall of the third ventricle) were excluded from the study.
Immunohistochemistry
Sections from the MPN were collected as above. Sections were incubated with antibody directed against MOR (1:24,000; Neuromics, Edina, MN) with 0.5% Triton X-100, 1% BSA, and 1% normal goat serum in PBS for 2 nights at 4 C. Sections were washed before being incubated in biotinylated goat antirabbit secondary antibody (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. The sections were washed in Tris-buffered saline with 0.05% Tween-20 (TNT) before being incubated in streptavidin-horseradish peroxidase (TSA kit; PerkinElmer, Waltham, MA) for 30 min at room temperature. The sections were washed again in TNT. Finally, the sections were incubated in fluorescein (TSA kit; PerkinElmer) for 5 min. The sections were washed in TNT and Tris buffer before being mounted onto slides. The slides were allowed to dry on a slide warmer and coverslipped with Aqua Poly/Mount (PolySciences Inc., Warrington, PA)
MOR internalization
To quantify the internalization of MOR, pictures of the MPN were taken using a Zeiss Axioskop 2 equipped with epifluorescent illumination and an Axiocam CCD camera at ×360 magnification using Zeiss Axiovision (version 4.8; New York, NY). Pictures were converted to grayscale in Adobe (San Jose, CA) Photoshop and adjusted for brightness and contrast to remove the background staining. To obtain an estimate of relative internalization, OD was then quantified using Image J (version 1.44p; National Institutes of Health, Bethesda, MD) in the pixel inverter function as has been described previously (15). Briefly, a circle of about 60 μm was imposed on the MPN and a measurement was taken. The circle was then moved to an area of the image outside the MPN to determine the background was taken. The background measurement was subtracted from the MPN measurement to obtain the OD of MOR staining in the MPN alone. OD has been correlated with receptor internalization (15, 22).
Western blots
To verify CAV1 knockdown and loss of membrane ERα in the ARH, animals that had been infused with scrambled or CAV1 siRNA were anesthetized 30 min after the last EB injection and decapitated. The brain was rinsed in cold PBS and the ARH dissected on ice and put in homogenization buffer containing protease inhibitors (from the plasma membrane protein extraction kit; Abcam, Cambridge, MA). The tissue was homogenized using a dounce homogenizer and membrane proteins were extracted according to the manufacturer's protocol. After extraction, the whole population of membrane proteins was resuspended in radioimmunoprecipitation assay lysis buffer and protease inhibitors (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were then heated to 95 C for 5 min in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA). Protein was run on a 10% acrylamide gel and transferred to a polyvinyl difluoride membrane overnight. Membranes were blocked in 5% milk in 0.1% Tris-buffered saline-Tween 20 for 1 h before being transferred to primary antibody against ERα (MC-20; Santa Cruz Biotechnology), made in 5% milk. The membrane was incubated in primary antibody overnight at 4 C. The next day, membranes were washed and incubated in peroxidase-conjugated goat antirabbit secondary antibody (1:2000; Vector Laboratories, Burlingame, CA) for 1 h. To remove the antibodies, membranes were washed again for at least 1 h and developed using GE Healthcare ECL for 1 min before being exposed to film (GE Healthcare, Indianapolis, IN). To reprobe the membranes, antibodies were stripped (60 mm Tris-HCl, 2.4 mm Tris, 2% sodium dodecyl sulfate, 0.7% β-mercaptoethanol) at 55 C for 5–7 min. Membranes were rinsed in double-distilled H2O (500 ml), incubated in 5% milk for 1 h, and placed in primary antibody against CAV1 (1:1000; BD Biosciences, San Jose, CA) with 5% milk overnight. A horse antimouse secondary antibody (1:2000; Vector Laboratories) was used and visualized as above. Finally, membranes were stripped, as before, and incubated in 5% milk with primary antibody directed against flotillin-1 (1:1000; Abcam) and visualized as described. Flotillin was used both as a loading and experimental control for both because it is a membrane protein found in lipid rafts and is not affected by EB administration (6).
Statistics
All data are expressed as the mean ± sem. All data were analyzed by two-tailed t tests. Differences were considered significant at P < 0.05. Statistical analysis was conducted using GraphPad Prism 5 software (version 5.02; GraphPad Software, Inc., La Jolla, CA). The number of animals used in each experiment is specified in Results.
Results
Knockdown of CAV1 and membrane ERα
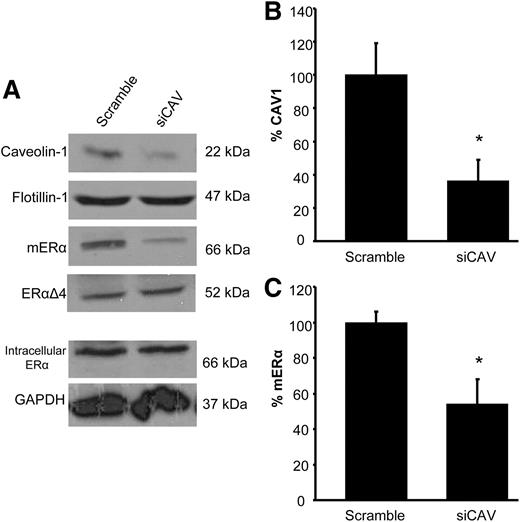
ovx female rats were injected every 4 d with 5 μg EB and were infused four times with siRNA directed against CAV1 or scrambled siRNA. The protocol was designed to knock down both endogenous and estradiol-induced levels of CAV1 mRNA and protein. In the ARH, CAV1 siRNA reduced membrane caveolin protein by 64% compared with scrambled siRNA controls (Fig. 1B; 100 ± 19.2 vs. 36.1 ± 13.0%; P = 0.0237, t = 2.878, df = 7; n = 4–5).
siRNA reduced CAV1 and ERα on the membrane. A, Western blots illustrating the loss of CAV1 and ERα in ARH membrane fractions after CAV1 siRNA Flotillin, an integral membrane protein, was not affected by CAV1 knockdown and was used to normalize the results. The ERα splice variant, ERαΔ4, which is found only on the membrane, and intracellular ERα levels were unaffected by CAV1 knockdown. Intracellular ERα was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). CAV1 siRNA reduced CAV1 by approximately 64% (B) and ERα by approximately 50% (C) compared with scrambled controls. *, P < 0.05 vs. scrambled siRNA (n = 4–6).
Because CAV1 was suspected to be the scaffold protein responsible for trafficking ERα to the cell membrane, we also examined the levels of ERα in the membrane fraction. Indeed, the loss of CAV1 affected the population of membrane ERα proteins but did not affect intracellular levels (Fig. 1A; intracellular ERα: 100 ± 25.2 vs. 107.5 ± 4.3%). As previously reported, the cell membrane fraction contained two ERα immunoreactive proteins, the full-length, 66-kDa ERα and the 52-kDa ERαΔ4 alternatively splices protein (18, 19, 23). Interestingly, unlike in primary cultures of hypothalamic neurons or astrocytes, in this ex vivo experiment, full-length ERα was more abundant on the membrane than the ERαΔ4, as demonstrated by the longer exposure times needed to see the 52-kDa splice variant. Although both were decreased, only the reduction in the full-length ERα was significant (Fig. 1C; mouse ERα: 100 ± 5.9 vs. 53.9 ± 14.1%; P = 0.0209, t = 2.793, df = 9; n = 5–6; ERαΔ4: 100 ± 9.2 vs. 77.1 ± 15.4%)
Knockdown of CAV1 affects MOR internalization and sexual receptivity
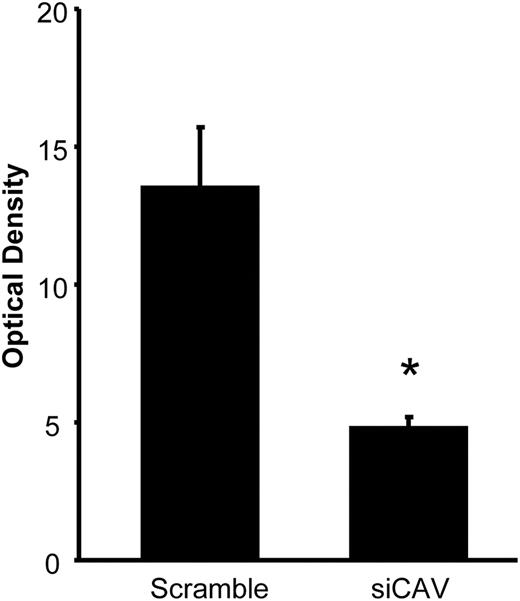
A hallmark of estradiol activation of the circuit that regulates sexual receptivity is the activation/internalization of MOR in the MPN (17, 22). MOR internalization is a consequence of estradiol membrane signaling (14, 15) that activates a microcircuit in the ARH that ultimately stimulates the release of β-END into the MPN. β-END causes rapid activation and internalization of MOR in the MPN (24). To determine whether blocking ERα trafficking prevents circuit activation MOR internalization, animals killed 30 min after the final EB injection were immunostained for MOR. Estradiol-induced MOR internalization was observed in scrambled siRNA-treated rats, but in CAV1 siRNA-treated rats, MOR internalization was reduced (Fig. 2; P = 0.0116, t = 3.257, df = 8; n = 4–6).
Knockdown of CAV1 attenuated MOR internalization. CAV1 siRNA microinfused into the ARH reduced MOR activation/internalization in the MPN, a measure of lordosis circuit activation, which requires estradiol membrane signaling. *, P < 0.05 vs. scrambled siRNA (n = 4–6).
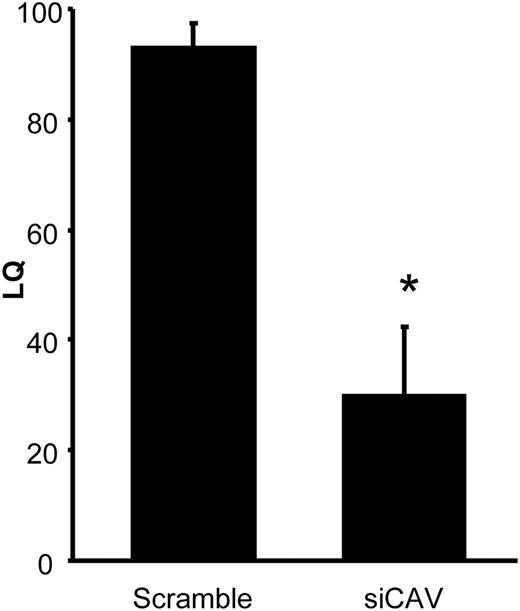
Transient MOR signaling is required for the full display of female sexual receptivity 30–48 h after EB treatment (24, 25). When CAV1 siRNA-treated rats were tested for lordosis behavior 30 h after the final EB injection, sexual receptivity was attenuated (Fig. 3; LQ: 93.3 ± 4.4 vs. 30.0 ± 12.3; P = 0.0004, t = 5.745, df = 8; n = 4–6).
Sexual receptivity is diminished when CAV1 is knocked down. LQ of female rats tested with stimulus males 30 h after their final EB injection. Control females, treated with scrambled siRNA, were fully sexually receptive. Sexual receptivity of those treated with CAV1 siRNA was significantly diminished. *, P < 0.05 vs. scrambled siRNA (n = 4–6).
Discussion
The major finding of the present study was that disruption of a proposed mechanism for ERα trafficking resulted in a loss of this receptor on the cell membrane. All of the effects of estradiol membrane signaling were abrogated. The CAV1 knockdown resulted in the loss of MOR activation and attenuation of female sexual receptivity. To our knowledge this was the first study to demonstrate the physiological relevance of CAV1 in maintaining a population of ERα on the cell membrane.
Caveolins may play several roles in neurons including trafficking receptors, mediating receptor-receptor interactions, and segregating signaling microdomains (reviewed in Ref. 26). It appears that with regard to ERα, caveolins may be performing all three functions. As shown in this study, caveolins are responsible for the transport of ERα to the membrane because knockdown of CAV1 resulted in a decrease in the population of membrane ERα. This is not due to a loss of ERα but a deficit of trafficking because intracellular ERα levels were not impacted by CAV1 siRNA treatment. Caveolin proteins mediate ERα association with specific mGluR on the membrane (6). This association allows the ERα transactivation of mGluR initiating G protein signaling; in this way a receptor that is not a G protein-coupled receptor induces cell signaling cascades. Finally, caveolins specify which mGluR is associated with ERα leading to facilitative or inhibitory signaling [(6); reviewed in Ref. 7]. Whether estradiol facilitates or inhibits downstream signaling is dependent on which specific mGluR is associated with ERα. Estradiol binding to ERα transactivates mGluR1a to initiate G protein-dependent signaling. In hypothalamic cells this activation of mGluR1a leads to the activation of the MAPK pathway or the release of inositol 1,4,5-triphosphate receptor-sensitive calcium stores. The association with mGluR is mediated by specific caveolin proteins (6).
In the present experiments, using a circuit in which cell signaling was dependent on ERα-mGluR1a, we confirmed that CAV1 was responsible for establishing the ERα-mGluR1a signaling unit (14–16). Antagonism of mGluR1a blocked estradiol membrane-initiated downstream cell signaling, morphological plasticity, and activation of the ARH-MPN circuit (8, 9, 14–16).
Although the loss of membrane ERα was only approximately 50%, it significantly impacted the ARH-MPN circuit as demonstrated by both the attenuation of MOR internalization and sexual receptivity. The present results confirm that membrane-initiated estradiol signaling is an important component of estradiol facilitation of lordosis behavior. The loss of the ERα-mGluR1 interaction through the inhibition of mGluR1 resulted in a reduction of estradiol-mediated signaling and ultimately the attenuation of female sexual behavior (15). Here we found that a reduction of the membrane ERα population results in the same deficits.
Although our experiments were focused on the ARH to MPN circuit and sexual receptivity, estradiol in the ARH affects many other systems. Estradiol-mediated spinogenesis in the ARH is at least partially regulated by the ERα-mGluR1 interaction (16). Another estradiol-sensitive circuit in the ARH regulates energy balance (27). How reducing membrane ERα signaling will affect these and other circuits will require additional experiments.
Surface biotinylation experiments done to identify the location of ERα within the membrane revealed that this receptor has an extracellular portion protruding from the cell membrane (18, 19, 23). In addition to the full-length, 66-kDa ERα, another, more abundant immunoreactive protein was identified. This approximately 52-kDa immunoreactive ERα was observed in primary cultures of neurons and astrocytes. Using primary cultures of embryonic and immortalized hypothalamic neurons (N-38), which express the 52-kDa ERα, we identified an mRNA missing exon 4 of ESRI. This ERαΔ4 mRNA previously had been identified in brain as an abundant alternatively spliced variant (28). In the present ex vivo study, ERαΔ4 was not as abundant as ERα. This difference does not appear to be a developmental phenomenon because greater membrane levels of ERαΔ4 were found in embryonic neurons and adult astrocytes. However, the greater ERα relative abundance may reflect a difference between cells in vitro compared with ex vivo (18, 19). Previous coimmunoprecipitation demonstrated an mGluR1a interaction with the full-length, 66-kDa ERα and not the ERαΔ4. Although we still do not know its function, ERαΔ4 trafficking and internalization parallels ERα: estradiol transiently increases cell membrane levels and its internalization. Further experiments will be needed to address these questions.
The current study highlighted another distinction between the ERα and ERαΔ4: CAV1 knockdown significantly reduced cell membrane ERα but not ERαΔ4 levels. There may be several potential explanations. First, CAV1 siRNA was a partial knockdown, reducing CAV1 protein only by half, and this may not have been sufficient to affect ERαΔ4 trafficking. Second, CAV1 may not be the scaffold protein responsible for trafficking ERαΔ4. Third, exon 4 codes for the nuclear translocation signal; perhaps ERαΔ4 is not as dependent on CAV1 for trafficking as the ERα. Future experiments will address these possibilities.
In summary, specific CAV1 knockdown in the ARH reduced levels of ERα due to a loss of CAV1-mediated trafficking to the cell membrane. The loss of membrane ERα in vivo resulted in deficits of circuit activation, demonstrated by an attenuation of MOR internalization and loss of lordosis behavior. The present results paralleled attenuating estradiol activation by blocking of mGluR1a in the ARH, suggesting an important role for CAV1 in maintaining the ERα-mGluR1a complex. CAV1 knockdown did not alter intracellular levels of ERα, further strengthening the case for membrane-initiated estradiol signaling in mediating sexual receptivity, a vital physiological function.
Acknowledgments
This work was supported by National Institutes of Health Grants DA013185 (to P.M.) and HD007228 training grant (to A.C.).
Disclosure Summary: The authors have nothing to declare.
Abbreviations
- ARH
Arcuate nucleus of the hypothalamus
- CAV1
caveolin-1
- EB
17β-estradiol benzoate
- β-END
β-endorphin
- ER
estrogen receptor
- LQ
lordosis quotient
- mGluR
metabotropic glutamate receptor
- MOR
μ-opioid receptor
- MPN
medial preoptic nucleus
- ovx
ovariectomized
- siRNA
small interfering RNA
- TNT
Tris-buffered saline with Tween-20.
References