-
PDF
- Split View
-
Views
-
Cite
Cite
Matthew C Poling, Elena Y Luo, Alexander S Kauffman, Sex Differences in Steroid Receptor Coexpression and Circadian-Timed Activation of Kisspeptin and RFRP-3 Neurons May Contribute to the Sexually Dimorphic Basis of the LH Surge, Endocrinology, Volume 158, Issue 10, 1 October 2017, Pages 3565–3578, https://doi.org/10.1210/en.2017-00405
Close - Share Icon Share
Abstract
In rodents, the ovulation-inducing luteinizing hormone (LH) surge is sexually dimorphic, occurring only in females, but the reasons for this sex difference are unclear. Two neuropeptides, kisspeptin and RFamide-related peptide 3 (RFRP-3), are hypothesized to regulate the gonadotropin-releasing hormone (GnRH)/LH surge. In females, both of these systems show circadian changes coincident with the LH surge, but whether males show similar temporal changes under comparable hormonal conditions is unknown. Here, we evaluated circadian time (CT)–dependent changes in gene expression and neuronal activation of Kiss1 and Rfrp neurons of female and male mice given identical LH surge-inducing estrogen regimens. As expected, females, but not males, displayed a late afternoon LH surge and GnRH neuronal activation. Kiss1 expression in the anteroventral periventricular nucleus (AVPV) was temporally increased in females in the late afternoon, whereas males demonstrated no temporal changes in AVPV Kiss1 expression. Likewise, neuronal activation of AVPV Kiss1 neurons was dramatically elevated in the late afternoon in females but was low at all circadian times in males. Estrogen receptor α levels in AVPV Kiss1 neurons were sexually dimorphic, being higher in females than males. AVPV progesterone receptor levels were also higher in females than males. Hypothalamic Rfrp messenger RNA levels showed no CT-dependent changes in either sex. However, Rfrp neuronal activation was temporally diminished in the afternoon/evening in females but not males. Collectively, the identified sex differences in absolute and CT-dependent AVPV Kiss1 levels, AVPV sex steroid receptor levels, and circadian-timed changes in neuronal activation of both Kiss1 and Rfrp neurons suggest that multiple sexually dimorphic processes in the brain may underlie proper LH surge generation.
Ovulation in female mammals is gated at the neuroendocrine level by an estrogen-mediated positive feedback induction of luteinizing hormone (LH) secretion. This “surge” release of LH is controlled by a preceding surge in gonadotropin-releasing hormone (GnRH) secretion from the brain. The secretion of GnRH is itself tightly regulated by a collection of upstream neurotransmitters and neuropeptides (1, 2). In rodents, the estrogen-induced GnRH/LH surge is circadian timed, occurring only in the late afternoon/early evening of proestrus, but not at other times of the day (3). The neural and molecular mechanisms that underlie the circadian and estrogen-dependent nature of the LH surge still remain incompletely characterized (4, 5), but are thought to include temporal and hormonal gating controlled by two hypothalamic neuropeptide systems, kisspeptin (encoded by Kiss1) and RFamide-related peptide 3 (RFRP-3, encoded by Rfrp).
Kisspeptin, acting on its receptor, Kiss1r, is an essential regulator of the reproductive axis. Humans or rodents with mutations in the Kiss1 or Kiss1r genes suffer from hypogonadotropic hypogonadism, impaired sexual development, and infertility (6–9). Kisspeptin directly acts on GnRH neurons (10–12), evidenced by the fact that kisspeptin stimulates both c-fos induction (a marker of neuronal activation) and electrical firing of GnRH neurons in brain explants (10, 13–15) and, subsequently, a robust secretion of gonadotropins (9, 15–19). Within the rodent brain, Kiss1 is expressed in two primary neuronal populations: the hypothalamic continuum of the anteroventral periventricular nucleus (AVPV) and neighboring rostral periventricular nucleus (PeN), and more caudally, the arcuate nucleus (20, 21). AVPV/PeN kisspeptin neurons in female rodents have been shown to be circadian regulated when estrogen is present, with Kiss1 messenger RNA (mRNA) and Kiss1 neuronal activation being significantly elevated just prior to and during the beginning of the dark phase, aligning with the occurrence of the LH surge (22). AVPV/PeN kisspeptin neurons receive input from the suprachiasmatic nucleus (SCN), the central circadian clock, and can be stimulated exogenously by neuropeptides expressed in the SCN (23, 24).
In contrast to kisspeptin, the neuropeptide RFRP-3 has potent inhibitory actions on LH secretion and is considered the mammalian ortholog of the avian gonadotropin-inhibiting hormone (25–30). In rodents, RFRP-3 may directly inhibit GnRH neuron activity (29) and/or GnRH secretion (31), or may act indirectly via kisspeptin neurons (32), thereby inhibiting LH secretion (33, 34). In the rodent brain, RFRP-3 cell bodies are found exclusively in and around the dorsal-medial nucleus of the hypothalamus (DMN), as determined by immunohistochemistry and in situ hybridization (11, 26, 33, 35, 36). With regard to the preovulatory LH surge, in female hamsters, RFRP-3 neurons show decreased immunoreactivity and decreased neuronal activation (assessed with c-fos induction) during the late afternoon of proestrus, coinciding with increased GnRH neuronal activation and the endogenous LH surge (37). Thus, it has been speculated that the increase in GnRH neuronal activation driving the LH surge could be due, in part, to an attenuation of upstream suppressive influence by RFRP-3. This possibility is supported by the finding that high estrogen levels, as would occur during estrogen positive feedback and the LH surge, moderately suppress Rfrp mRNA expression in rodents (11) and RFRP-3 receptor knockout mice have larger litters (34).
In rodents, the LH surge mechanism is sexually differentiated, with only females being able to generate LH surges in response to sex steroid–priming in adulthood. Indeed, adult male rodents given appropriate estradiol or estradiol plus progesterone paradigms fail to generate LH surges, indicating their reproductive brain circuitry is different in one or more ways from that of females. However, the underlying mechanisms of this sex difference in LH surge capability are still poorly understood. Importantly, as with the LH surge, the AVPV/PeN population of Kiss1 neurons is sexually dimorphic, with female rodents having considerably more Kiss1 neurons and more Kiss1 mRNA in each cell than males (38). Thus, one common postulation is that the sexually dimorphic ability to generate LH surges simply reflects the total amount of kisspeptin being greater in females than males. Although this is likely a key facet of the LH surge sex difference, it remains to be determined if males demonstrate additional sex differences in other parameters relating to the LH surge, such as circadian-timed increases in Kiss1 levels or kisspeptin neuronal activation, circadian-timed decreases in the RFRP-3 system, or coexpression levels of sex steroid receptors.
This study was designed to investigate changes in kisspeptin and RFRP-3 neurons in mice with regard to the sexually dimorphic induction of the LH surge. We compared male and female mice that were treated with a similar exogenous estrogen regimen known to elicit LH surges and determined (1) whether the circadian increase of AVPV/PeN Kiss1 mRNA levels and/or Kiss1 neuronal activation is sexually dimorphic; (2) whether sex differences in estrogen receptor α (ERα) or progesterone receptor (PR) levels in the AVPV/PeN or coexpression in AVPV/PeN Kiss1 neurons exist that may account, in part, for sex differences in Kiss1 neuron circadian changes; and (3) whether hypothalamic Rfrp expression and/or Rfrp neuronal activation in mice exhibit temporal changes coincident with the circadian-timed LH surge and, if so, does this occur similarly in both sexes.
Materials and Methods
Animals
Young adult (7 to 8 weeks old) C57BL6 mice of both sexes were singly-housed on a 12-12 light-dark cycle (lights off at 5:00 pm) with food, water, and in-cage running wheel available ad libitum for the entire study, matching the housing setup of our previous study (22). The running wheels were hooked up to computers to continuously measure daily circadian changes in locomotor activity (not shown). All experiments were conducted in accordance with the National Institutes of Health Animal Care and Use Guidelines and with approval of the Animal Care and Use Committee of the University of California, San Diego.
Hormonal treatment and tissue collection
After 7 to 10 days of acclimatization to the cages with running wheels, mice were anesthetized with isoflurane and bilaterally gonadectomized. Mice were then subcutaneously implanted with a SILASTIC brand (Dow Corning, Midland, MI; inner diameter, 0.20 cm; outer diameter, 0.318 cm) capsule containing either 0.750 µg (for females) or 0.885 µg (for males, because of their higher body weight) of 17-β estradiol (E2) dissolved in sesame oil (22, 39). Previous studies in female mice by our laboratory and others, and pilot experiments in male mice, determined that this hormone treatment produces similar constant elevated E2 levels of ∼18 to 24 pg/mL in both sexes, similar to female mouse proestrus levels, and induces a robust circadian-timed LH surge in females 2 days later, around the time of lights off (22, 39, 40).
Two days after E2 capsule implantation, all mice were briefly anesthetized with isoflurane and immediately euthanized by rapid decapitation at one of five circadian time (CT) points: CT4, CT10.5, CT12, CT13.5, and CT18 (n = 7 to 10 mice/sex/time point), with CT 12 representing the time of lights off (5:00 pm) by standard chronobiology convention. In this context, CT4 and CT12 correspond to baseline morning LH and peak LH surge levels, respectively. CT10.5 and CT13.5 bracket 90 minutes on either side of the peak of the LH surge to study the onset and offset periods of the surge event. CT18 represents a late-night time period after the LH surge has ended, when LH levels and GnRH neuronal activity have returned to baseline morning values. At euthanization, blood and brains were collected. Brains were immediately frozen on dry ice and stored at −80°C until being processed with in situ hybridization (ISH). Blood was centrifuged 90 minutes after collection and the serum stored at −20°C until assaying for hormone levels. Euthanizations that occurred at time points during lights off (e.g., CT13.5, CT18) were performed under red-light illumination.
Hormone measurements
All hormone measurements were run in singlet and performed by the University of Virginia Ligand Assay Core. Blood serum collected was measured for LH levels using a sensitive mouse LH radioimmunoassay, as in previous studies (22, 39–42). Serum was also tested for E2 levels to confirm elevated circulating E2 in both sexes. Serum E2 was measured by enzyme-linked immunosorbent assay, as in previous reports (39).
Single-label ISH
Five coronal series of 20-µm brain sections were cut on a cryostat, thaw-mounted onto Superfrost-plus slides, and stored at −80°C until use in ISH. The Kiss1 (20), Rfrp (11), ERα (11), PR (42), Gnrh (39), and cfos (43) complementary RNA ISH riboprobes have been described and validated previously. Single-label ISH was performed as previously described (11, 39). In brief, slide-mounted sections encompassing the entire AVPV/PeN or DMN were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× sodium citrate, sodium chloride (SSC), delipidated in chloroform, dehydrated in ethanols, and air-dried. Radiolabeled (33P) Kiss1, Rfrp, ERα, or PR antisense riboprobe (0.04 pmol/mL) was combined with transfer RNA, heat-denatured, added to hybridization buffer, and applied to each slide (100 μL/slide). Slides were cover-slipped and placed in a 55°C humidity chamber overnight. The slides were then washed in 4× SSC, treated with RNAse A treatment at 37°C, washed in 2× SSC at room temperature, and washed in 0.1× SSC at 62°C before being dehydrated in ethanols and air-dried. Slides were then dipped in Kodak NTB emulsion, air-dried, and stored at 4°C for 3 to 4 days (depending on the assay) before being developed and cover-slipped.
Double-label ISH
Double-label ISH was performed as previously described (32). Briefly, slide-mounted brain sections encompassing the preoptic area, AVPV/PeN, or DMN were fixed in 4% paraformaldehyde; pretreated with acetic anhydride, rinsed in 2× SSC; delipidated in chloroform; dehydrated in ethanols; and air-dried. Radiolabeled (33P) antisense cfos or ERα (0.05 pmol/mL) and digoxigenin (DIG)-labeled Gnrh, Kiss1, or Rfrp riboprobes (Roche Digoxigenin labeling kit, 1:500) were combined with transfer RNA, heat denatured, dissolved together in hybridization buffer applied to slides (100 μL/slide), and hybridized at 55°C overnight. The next day, slides were washed in 4× SSC, treated with RNAse A at 37°C, and subsequently washed in 0.1× SSC at 62°C. Slides were then incubated in 2× SSC with 0.05% Triton X-100 containing 3% normal sheep serum for 75 minutes at room temperature and then incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase (1:500; Roche). The next day, slides were washed and then incubated with Vector Red alkaline phosphatase substrate (Vector Laboratories, CA) for 1 hour at room temperature. Slides were then air-dried, dipped in Kodak NTB emulsion, stored at 4°C, and developed and cover-slipped 7 to 9 days later (depending on the assay).
ISH quantification and analyses
ISH slides were analyzed with an automated image processing system (Dr. Don Clifton, University of Washington) by a person unaware of the treatment group of each slide (44). For single-label assays, the software counted the number of ISH silver grain clusters representing Kiss1, Rfrp, ERα, or PR cells as well as the number of silver grains in each cell (a semiquantitative index of Kiss1, Rfrp, ERα, or PR mRNA expressed per cell) (45–47). Cells were considered Kiss1 or Rfrp positive when the number of silver grains in a cluster exceeded that of background by threefold. For double labels, DIG-containing cells (Gnrh, Kiss1, or Rfrp cells) were identified under fluorescence microscopy, and the grain-counting software was then used to quantify silver grains (representing cfos or ERα mRNA) overlying each cell in dark field. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its ratio was >3.
Statistical analysis
All data are expressed as the mean ± standard error of the mean for each group. In all experiments, differences were analyzed by Student t test or two-way analysis of variance (ANOVA), followed by post hoc comparisons for individual groups via Fisher’s (protected) least significant difference. Statistical significance was set at P < 0.05. All analyses were performed in Statview 5.0.1 (SAS Institute, Cary, NC).
Results
Circadian increases in LH levels and GnRH neuronal activation in females but not males
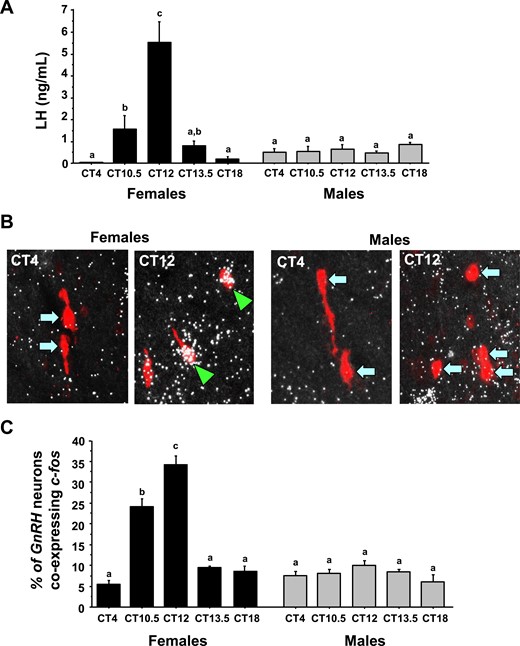
ANOVA analysis determined that serum LH levels showed significant main effects for both CT (P < 0.01) and sex (P < 0.01) and a significant CT-by-sex interaction (P < 0.01). In E2-treated females, serum LH values were nearly undetectable in the morning at CT4, increased significantly in the late afternoon at CT10.5 and CT12, and then fell again in the early evening at CT13.5 (Fig. 1). LH was again near undetectable baseline levels by the night at CT18. In contrast, as expected, E2-treated males showed no circadian changes in serum LH (Fig. 1), with LH values at all time points being similar to morning CT4 values.
Circadian-timed changes in LH and cfos induction in GnRH neurons of gonadectomized and estrogen-replaced animals. (A) Serum LH of gonadectomized and estrogen-replaced female and male mice. Female mice show statistically significant circadian-timed increase in serum LH, occurring shortly before and during the dark phase, whereas males show no differences in serum LH at any time. (B) Representative photomicrographs of cfos (a marker of neuronal activation, silver grains) colocalizing with Gnrh neurons (red fluorescence) of both males and females at two circadian time points (all mice are gonadectomized and had estrogen replaced). Green arrowheads, Gnrh cells with cfos; blue arrows, example Gnrh cells lacking cfos expression. (C) Quantification of the percent colocalization of cfos in Gnrh neurons. Different lowercase letters indicate significantly different groups (P < 0.05).
The pattern of cfos induction (a marker of neuronal activation) in GnRH neurons matched serum LH values, as expected. ANOVA revealed that GnRH-cfos coexpression showed significant main effects for both CT (P < 0.01) and sex (P < 0.01), and also a significant CT-by-sex interaction (P < 0.01). E2-treated females displayed high levels of cfos coexpression in GnRH neurons at the height of the LH surge (CT12) but low cfos-GnRH expression at CT4 and CT18 when LH was virtually undetectable (Fig. 1B and 1C). Conversely, E2-treated males showed no elevated cfos-GnRH coexpression at any time point and there were no CT-dependent changes in cfos induction in GnRH neurons of males (Fig. 1B and 1C).
Circadian increases in AVPV/PeN Kiss1 mRNA levels are sexually dimorphic
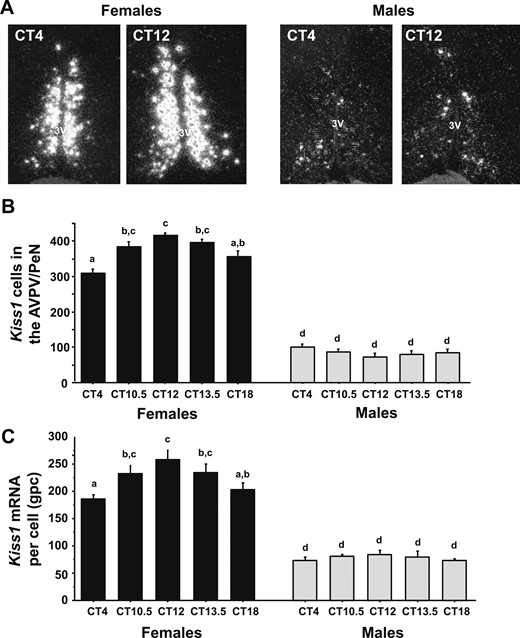
To determine if there is a sex difference in the circadian-timed upregulation of Kiss1 expression in the AVPV/PeN during an LH surge, single-label ISH was used to measure Kiss1 mRNA levels in the AVPV/PeN of E2-treated males and females throughout the day, with specific emphasis on times before and during the LH surge. ANOVA determined that AVPV/PeN Kiss1 levels showed significant a main effect of sex, as previously reported (38), with E2-treated females having more Kiss1 cells (P < 0.01) and higher Kiss1 mRNA per cell (P < 0.01) than E2-treated males. ANOVA also revealed that AVPV/PeN Kiss1 cell numbers showed a significant main effect of CT (P < 0.01) and a significant CT-by-sex interaction (P < 0.01). E2-treated female mice showed a substantial temporal increase in the number of Kiss1 neurons during the LH surge (CT12), relative to baseline values at CT4, whereas E2-treated males showed no CT-dependent changes in Kiss1 cell number (Fig. 2A–2C), P < 0.01]. Similarly, there was a significant main effect of CT for Kiss1 mRNA levels per cell (P < 0.05), although there was no significant sex-by-CT interaction for this measure (Fig. 2A–2C).
ISH for Kiss1 mRNA in the AVPV/PeN during the time of the LH surge. (A) Representative photomicrographs of Kiss1 mRNA in the AVPV/PeN in both males and females at two time points. Female mice euthanized at CT12 have considerably more Kiss1 mRNA than CT4 females or males at either time point. All animals are gonadectomized and estrogen replaced. (B) Quantification of the number of Kiss1 neurons in the AVPV/PeN. (C) Quantification of the grains per cell (a semiquantitative measure of mRNA) of Kiss1 neurons in the AVPV/PeN. Different lowercase letters indicate significantly different groups. 3V, third ventricle; gpc, grains per cell.
Circadian increases in AVPV/PeN Kiss1 neuronal activation occur in females but not males
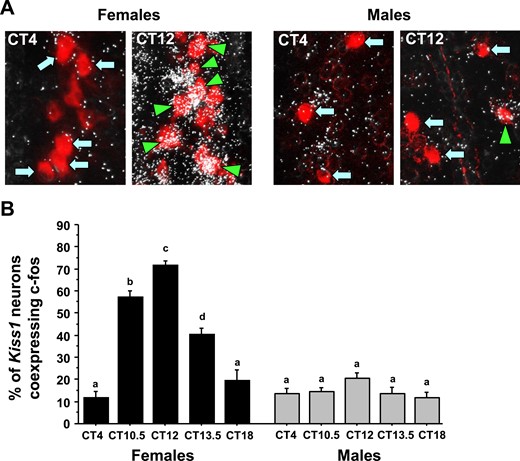
To determine if there is a sex difference in the circadian increase in neuronal activation of AVPV/PeN Kiss1 neurons, double-label ISH was used to measure cfos induction in AVPV/PeN Kiss1 neurons of E2-treated males and females throughout the day. ANOVA revealed that Kiss1-cfos coexpression levels showed significant main effects for both sex (P < 0.01) and CT (P < 0.01), and also a significant CT-by-sex interaction (P < 0.01). E2-treated female mice showed a significant CT-dependent increase in Kiss1 neuronal activation (cfos/Kiss1 coexpression) that occurred in synchrony with the timing of the LH surge and heightened GnRH neuronal activation (CT10.5 and CT12 vs CT4; P < 0.01; Fig. 3). In contrast, E2-treated males failed to show marked increases in Kiss1 neuronal activation at any circadian time point, with cfos/Kiss1 coexpression being similar throughout the day.
Double-label ISH for cfos colocalization in Kiss1 neurons during the time of the LH surge. (A) Representative photomicrographs of cfos (silver grains) colocalizing with AVPV/PeN Kiss1 neurons (red fluorescence) in E2-treated males and females at two time points. Kiss1 neurons in female mice euthanized at CT12 have substantially more cfos colocalization than at CT4; male Kiss1 neurons have low cfos induction at both time points. Green arrowheads, example Kiss1 cells with cfos; blue arrows, example Kiss1 cells lacking cfos expression. (B) Quantification of the percent colocalization of cfos in AVPV/PeN Kiss1 neurons in each sex across the circadian day. Different lowercase letters indicate significantly different groups (P < 0.05).
ERα and PR levels in the AVPV/PeN are higher in females than males
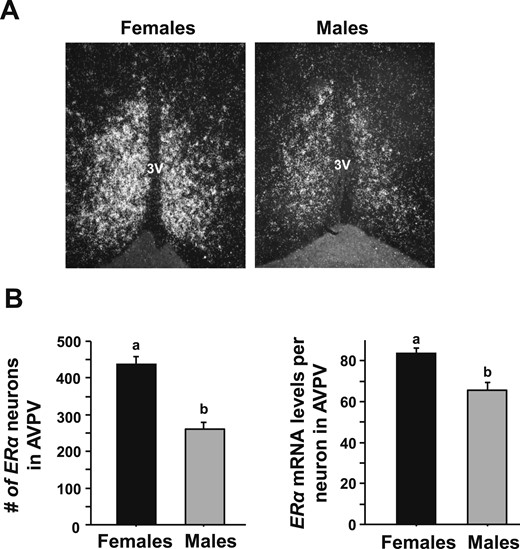
To determine if the sex differences in CT-dependent Kiss1 gene expression and Kiss1 neuronal activation might be due, in part, to sex differences in E2 signaling, single-label ISH for ER levels was performed on brains from both sexes of E2-treated mice at the CT12 time point (when the LH surge is maximal). E2-treated females expressed significantly greater numbers of cells expressing ERα mRNA in the AVPV/PeN than E2-treated males (Fig. 4,P < 0.05). Furthermore, the mean relative amount of ERα mRNA in each AVPV/PeN neuron was significantly less in E2-treated males than E2-treated females (Fig. 4, P < 0.05).
Single-label ISH for ERα mRNA expression in AVPV/PeN in male and female mice. (A) Representative photomicrographs of ERα in the AVPV/PeN in E2-treated male and female mice at CT12. Female mice have a considerably higher degree of ERα in the AVPV/PeN than do males. (B) Quantification of the gene expression of ERα in the AVPV/PeN of E2-treated males and females. Different lowercase letters indicate significantly different groups (P < 0.05).
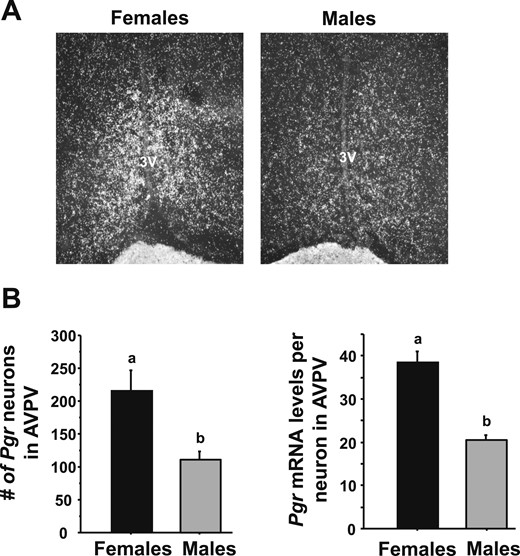
Previous findings from our laboratory and others have demonstrated that PR signaling in the AVPV (48), including specifically in kisspeptin neurons (42), is critical for proper LH surge induction in female rodents. We therefore next tested whether E2-treated males and females display different levels of PR in the AVPV region, because such a sex difference could be one potential mechanism underlying sex differences in circadian Kiss1 levels or Kiss1 neuronal activation. We found that, although both sexes expressed PR in the AVPV region, such expression was significantly greater in females than males (Fig. 5). Specifically, E2-treated females had many more detectable PR neurons and greater PR mRNA levels per neuron in the AVPV than did E2-treated males (P < 0.05 for both measures, Fig. 5).
Single-label ISH for PR gene expression in AVPV/PeN in male and female mice. (A) Representative photomicrographs of progesterone receptor gene (Pgr) in the AVPV/PeN in E2-treated male and female mice at CT12. Female mice have a considerably higher degree of Pgr in the AVPV/PeN than do males. (B) Quantification of the expression of Pgr in the AVPV/PeN of E2-treated males and females. Different lowercase letters indicate significantly different groups (P < 0.05).
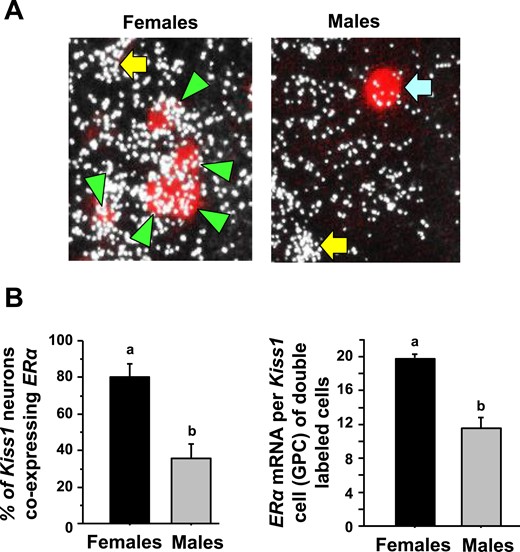
To determine if the lower ERα levels in the AVPV/PeN in males were occurring in kisspeptin neurons specifically, double-label ISH for ERα levels in Kiss1 neurons was performed on brains from both sexes of E2-treated mice at the CT12 time point. We found that >80% of AVPV/PeN Kiss1 neurons in E2-treated females express ERα mRNA, whereas only ∼40% of AVPV/PeN Kiss1 neurons in E2-treated males express ERα (Fig. 6, P < 0.05). Furthermore, the relative amount of ERα mRNA in each Kiss1 neuron that showed coexpression was significantly less in males than females (Fig. 6, P < 0.05).
Double-label ISH for ERα coexpression in AVPV/PeN Kiss1 neurons in male and female mice. (A) Representative photomicrographs of ERα mRNA colocalizing with AVPV/PeN Kiss1 neurons in E2-treated male and female mice at CT12. Green arrowheads, example Kiss1 cells with ERα; blue arrow, example Kiss1 cells lacking ERα expression; yellow arrow, ERα cell that is not a Kiss1 cell. (B) Quantification of the percent coexpression of ERα in AVPV/PeN Kiss1 neurons of E2-treated males and females. Female mice have a considerably higher degree of Kiss1 + ERα coexpression than do males. Different lowercase letters indicate significantly different groups (P < 0.05). GPC, grains per cell.
Circadian decreases in Rfrp neuronal activation occur in females but not males
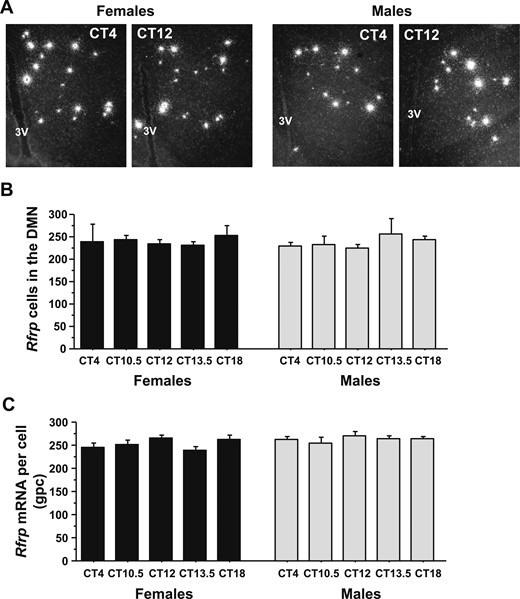
A previous study in female hamsters showed a circadian-timed reduction in both the number of detectable RFRP-3 neurons and RFRP-3 neuronal activation at the time of the endogenous LH surge on proestrus. However, similar temporal changes in RFRP-3 levels or neuronal activation have not been assessed in other species or in males. We therefore hypothesized that Rfrp gene expression and/or Rfrp neuronal activation is similarly decreased in female mice before or during the LH surge, thereby permitting increased GnRH/LH release. We also hypothesized that part of the sexually dimorphic nature of the LH surge may include sex differences in circadian changes of RFPR-3 neurons. To test these possibilities, we used ISH to examine DMN Rfrp mRNA levels and Rfrp-cfos coexpression in the brains of E2-treated mice of both sexes. ANOVA analysis revealed that Rfrp levels did not show a substantial main effect for either CT or sex or a substantial interaction. There were no CT-dependent differences in the number of Rfrp neurons or the amount of Rfrp mRNA per cell at any time point in E2-treated mice of either sex (Fig. 7A–7C), nor any sex difference in Rfrp levels in these E2-treated mice (Fig. 7A–7C).
ISH for Rfrp mRNA in the DMN before and during the time of the LH surge. (A) Representative photomicrographs of Rfrp mRNA in the AVPV/PeN in E2-treated males and females at two circadian times. (B) Quantification of the number of Rfrp neurons in the DMN of E2-treated male and female mice at different circadian times. (C) Quantification of the relative Rfrp mRNA per cell in E2-treated male and female mice across the circadian day. Different letters indicate significantly different groups (P < 0.05). 3V, third ventricle.
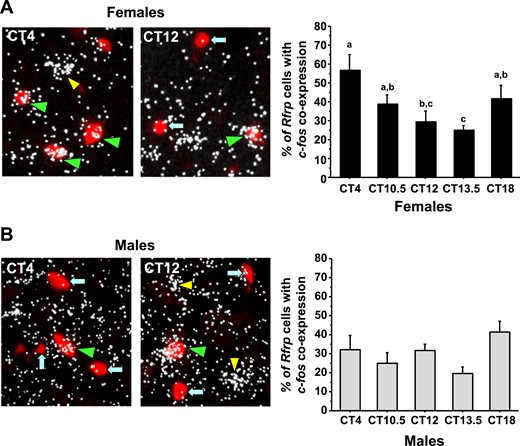
Using double-label ISH to study cfos-Rfrp coexpression, we next assessed Rfrp neuronal activation in both sexes across the circadian day. We identified a substantial temporal decrease in Rfrp neuronal activation during the height of, and toward the end of, the LH surge in E2-treated females (Fig. 8A) , P < 0.05]. In contrast, we found no temporal differences in Rfrp neuronal activation between any time point in E2-treated males, with Rfrp neuronal activation remaining constant in these mice throughout the day (Fig. 8B).
Double-label ISH for cfos colocalization in Rfrp neurons of mice during the time of the LH surge. (A) (Left) Representative photomicrographs of cfos (a marker of neuronal activation, silver grains) colocalizing with Rfrp neurons (red fluorescence) of E2-treated females mice at two circadian times. Green arrowheads, example Rfrp cells with cfos; blue arrows, example Rfrp cells lacking cfos expression; yellow arrowhead, example cfos-expressing cell that is not an Rfrp cell. (Right) Quantification of cfos induction in female Rfrp neurons at various circadian times. Female mice euthanized at CT12 have notably less cfos colocalization in Rfrp neurons than females euthanized at CT4. Different lowercase letters indicate significantly different groups (P < 0.05). (B) (Left) Representative photomicrographs of cfos colocalizing with Rfrp neurons of E2-treated male mice at two circadian times. See (A) for explanation of arrows and arrowheads. (Right) Quantitative analysis shows no major circadian differences in the degree of Rfrp neuronal activation in males.
Discussion
The LH surge in rodents is both circadian-timed and sexually differentiated, with only females being able to generate an LH surge in response to adulthood sex steroid priming; however, the neural and molecular mechanisms underlying the sexually dimorphic nature of the LH surge is not fully known. In this study, we demonstrated that, unlike in E2-primed females, the AVPV/PeN kisspeptin population of E2-primed males remains relatively unchanged throughout the day, despite similar elevated E2 exposure. Specifically, E2-treated female mice show substantial CT-dependent increases in both AVPV/PeN Kiss1 mRNA levels and neuronal activation of Kiss1 neurons during the late afternoon/early evening, coincident with the LH surge and GnRH neuronal activation, whereas E2-treated male mice fail to show notable CT-dependent changes in Kiss1 mRNA levels, Kiss1 neuronal activation, GnRH neuronal activation, or LH secretion. Furthermore, we report that female mice have a higher degree of ERα coexpression in Kiss1 AVPV/PeN neurons than do males, suggesting that the male AVPV/PeN Kiss1 system may be less sensitive to E2 signals. This sex difference in ERα correlates with a higher degree of expression of PR, a key player in the LH surge mechanism, in the AVPV/PeN of females than males. Last, we also show that, although there are no overt circadian changes in Rfrp mRNA expression in either sex, Rfrp neuronal activation is significantly diminished in female mice in the early evening, coincident with the peak of the LH surge. Conversely, Rfrp neuronal activation remained unchanged in male mice between the morning and evening. Collectively, these findings provide insights into the long-standing question of why the brains of adult females, but not males, are capable of generating a GnRH/LH surge in response to elevated E2 exposure.
The role of kisspeptin in stimulating the LH surge is unequivocal; several experiments have demonstrated that Kiss1 and Kiss1r null mice are unable to produce an LH surge under multiple hormonal paradigms (39, 49). Given that normal male rodents are unable to produce an LH surge, even after suitable sex steroid priming in adulthood, and that there is a striking sex difference in the number of Kiss1 cells in the AVPV/PeN (38), many investigators have assumed that male mice are unable to produce an LH surge simply because they have less kisspeptin in the AVPV/PeN than females. Although this sex difference in absolute kisspeptin levels is likely to be an important aspect of the LH surge sex difference, this assumption excludes the possibility of other sexually dimorphic factors also being involved, and overlooks the large potency of kisspeptin stimulation of GnRH that might be achieved even with only a few kisspeptin cells (in males). Our present findings establish that the AVPV/PeN kisspeptin system is also sexually dimorphic in its circadian-dependent changes in Kiss1 expression levels and Kiss1 neuronal activation, because male mice fail to show a noteworthy circadian increase in either Kiss1 mRNA levels or cfos-Kiss1 coexpression in the early evening as do females. Thus, these male kisspeptin cells are not only fewer in number but also fail to become “activated” in the early evening as do female kisspeptin cells. Moreover, as noted later, sex steroid receptor levels are also higher in female AVPV/PeN kisspeptin cells than in males. Thus, several factors associated with the AVPV/PeN Kiss1 system may be contributing to the sex difference in LH surge generation.
ERα necessity for LH surge generation is indisputable and is the primary estrogen receptor responsible for mediating the upregulation of Kiss1 expression in the AVPV/PeN (50). Furthermore, mice with conditional deletion of ERα from Kiss1 neurons fail to exhibit an LH surge (51). Previous reports have shown that 60% to 98% of female AVPV/PeN Kiss1 neurons express ERα (49, 50, 52), depending on the species and method of detection. However, ERα levels in male AVPV neurons have not previously been measured with regard to the time of LH surge. Our present results show that E2-treated male mice express ERα in their AVPV/PeN Kiss1 cells, but to a lesser degree than E2-treated females. Given ERα’s essential role in inducing Kiss1 expression and LH surge generation, the lower degree of ERα coexpression in male Kiss1 neurons may explain, in part, why male AVPV/PeN Kiss1 fail to show a large induction of cfos or a circadian upregulation of Kiss1 mRNA. Furthermore, we also demonstrated a sex difference in PR levels in the AVPV, with these levels being lower in males than females. AVPV PR signaling is critical for LH surge generation (48, 53), and female mice lacking PR exclusively in Kiss1 neurons fail to generate proper LH surges and show subfertility (42). Thus, the lower levels of AVPV PR observed in males may contribute to their lack of LH surge generation.
In both female mice and hamsters, arginine-vasopressin (AVP) fibers originating from the SCN innervate AVPV/PeN kisspeptin cell bodies (23, 24). Although this specific SCN-kisspeptin circuit has not yet been examined in male rodents, there are no reported sex differences in AVP fibers originating from the SCN (54–56); therefore, AVPV/PeN kisspeptin neurons of males may be innervated similarly as females. Female AVPV/PeN kisspeptin neurons express the AVP receptor, V1a, and increase their firing rate when stimulated with AVP, but again, similar experiments have not been reported for male AVPV/PeN kisspeptin cells (23, 24). Future studies addressing these issues in both sexes in detail will shed more light on potential sex differences in “upstream” circadian inputs into Kiss1 neurons.
RFRP-3’s known inhibitory effects on the reproductive axis led us to hypothesize that Rfrp expression would be lower during the LH surge, perhaps reflecting lessening of inhibition on GnRH secretion to permit a GnRH surge. Indeed, when given exogenously in rats, RFRP-3 can blunt the endogenous activation of GnRH neurons during E2-induced LH surges (28). Moreover, in Syrian hamsters, the number of detectable RFRP-3 immunoreactive cell bodies is lower during the endogenous proestrus LH surge and increases again to baseline levels after the LH surge is over (37). However, in the current study, we found that E2-treated female mice show no important CT-dependent changes in Rfrp mRNA expression before or during the LH surge, nor were there any CT-dependent changes in in Rfrp mRNA levels in similarly treated male mice. This suggests a possible species difference in the circadian control of Rfrp neurons. However, we did find that the activation status of Rfrp neurons, as measured by cfos coexpression, did show circadian changes in E2-treated female mice, decreasing during the LH surge, similar to the temporal pattern previously seen in proestrus Syrian hamsters (37). Interestingly, both our study and the previous report in hamsters show that the onset of the LH surge precedes the maximal circadian decrease in Rfrp neuronal activation. It may be that RFRP-3 neurons are not involved in the initial triggering of the surge but rather play a role in modulating or timing the peak secretion of the surge or the overall duration of the surge. In contrast, kisspeptin neurons show simultaneous neuronal activation in synchrony with the LH surge onset, suggesting they play a key role in initial triggering of the LH surge. It is also possible that the observed circadian-timed decrease of Rfrp neuron activation is coincidental but unrelated to the LH surge mechanism, an issue that requires additional future studies to tease apart. Yet, as noted later, this CT-dependent decrease in Rfrp neuron activation is sexually dimorphic, perhaps linking it to the LH surge. Last, the observed CT-dependent changes in Kiss1 gene expression in females may be compensatory mechanisms to replenish releasable kisspeptin peptide during and after the large surge release; in contrast, the absence of circadian changes in Rfrp levels may be because RFRP-3 release is being suppressed, rather than stimulated, and thus no compensatory mechanism is needed to replenish peptide stores.
We found that the circadian dampening of Rfrp neuron activation is sexually dimorphic because the Rfrp neurons of male mice, unlike females, showed no circadian changes in cfos coexpression. To date, this circadian change in Rfrp neuronal activation is the only known sex difference in Rfrp neurons because previous investigations have yielded no substantial effect of sex on Rfrp cell number, cell morphology, developmental pattern, or gene coexpression, including coexpression of ERα (11, 26, 32, 33). This Rfrp neuronal activation sex difference may be intrinsic to the Rfrp neuron itself, or Rfrp neurons may receive sexually dimorphic inputs from other brain regions, such as the SCN or interneurons linked to the SCN (57). The DMN is generally not considered a sexually dimorphic nucleus (56, 58), so an intrinsic sex difference in Rfrp neurons seems unlikely but remains possible. Further investigations will hopefully assess the presence or absence of sexually dimorphic inputs to Rfrp neurons.
In summary, the data presented here further our understanding of the sexually dimorphic nature of the circadian-timed LH surge. Although the sex difference in absolute AVPV/PeN kisspeptin levels has been assumed to be an important aspect of the LH surge sex difference, this assumption excludes the possibility of other sexually dimorphic factors also being involved. In fact, our present findings identify additional sexually dimorphic aspects of several reproductive neural populations involved in governing the LH surge. Unlike females, male mice, in addition to having fewer overall Kiss1-expressing neurons in the AVPV/PeN as previously shown, fail to show a circadian increase in Kiss1 expression levels or Kiss1 neuronal activation in response to E2 priming at positive feedback levels. This may be due to our finding that males have less ERα coexpression in AVPV/PeN Kiss1 neurons and less PR than do females. Moreover, although the amount of Rfrp mRNA produced in the DMN does not change before or during the LH surge in females or similarly treated males, the activation of Rfrp neurons is diminished during the peak of the LH surge in females, but not in males. Whether similar patterns, or lack thereof, occur with kisspeptin, RFRP-3, and sex steroid receptor protein levels was not determined and remains to be ascertained. Overall, our findings suggest that the inability of steroid-treated male rodents to demonstrate positive feedback induction of GnRH/LH secretion may reflect multiple neural parameters, including lower overall kisspeptin levels, absent circadian increases in Kiss1 expression, minimal Kiss1 neuron activation, lower levels of ERα and PR, and an absent circadian decrease in RFRP-3 neuron activation.
Abbreviations:
- ANOVA
analysis of variance
- AVP
arginine-vasopressin
- AVPV
anteroventral periventricular nucleus
- CT
circadian time
- DIG
digoxigenin
- DMN
dorsal-medial nucleus of the hypothalamus
- E2
17-β estradiol
- ERα
estrogen receptor α
- GnRH
gonadotropin-releasing hormone
- ISH
in situ hybridization
- LH
luteinizing hormone
- mRNA
messenger RNA
- PeN
rostral periventricular nucleus
- PR
progesterone receptor
- RFRP-3
RFamide-related peptide 3
- SCN
suprachiasmatic nucleus
- SSC
sodium citrate, sodium chloride.
Acknowledgments
Financial Support: This research was supported by National Science Foundation Grants IOS-1025893 and IOS-1457226, National Institutes of Health (NIH) Grant R01 HD082567, and NIH Grants P50 HD012303 (University of California, San Diego) and P50 HD28934 (University of Virginia Ligand Assay Core).
Disclosure Summary: The authors have nothing to disclose.
References