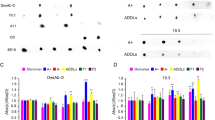
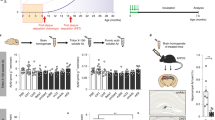
Abstract
Alzheimer’s disease (AD) is a fatal, progressive dementia for which there is no cure and for which a molecular basis has yet to be established. However, considerable evidence suggests that AD is linked to neurotoxic assemblies of the 42-amino-acid peptide amyloid β (Aβ). There is now a clear body of evidence that shows this neurotoxicity resides not only in insoluble fibrils of Aβ but also in soluble Aβ ADDLs (Aβ-derived diffusible ligands) and larger protofibrils. Further, anti-Aβ antibodies have been reported to reverse memory failure in human amyloid precursor protein (hAPP)-expressed transgenic mice in a manner that suggests symptom reversal is attributable to targeting of ADDLs. Clearly, a search for drugs targeting the assembly of these soluble Aβ species represents a new and potentially important approach to the treatment of AD. In this work we describe the development of a dot-blot immunoassay to measure ADDL at the femtomole level, its use in defining the time course of ADDL formation, and its use in determining the presence of ADDLs in the hAPP transgenic mouse brain. Discussion of a protocol to screen agents for inhibition of neurotoxic ADDL formation both in vivo and in vitro is also presented. The methods are suitable for screening combinatorial libraries and, importantly, provide the potential for simultaneous information on candidate transport across the blood-brain barrier.
Similar content being viewed by others
References
Bohrmann B., Adrian M., Dubochet J., Kuner P., Muller F., Huber W., et al. (2000) Self-assembly of beta-amyloid 42 is retarded by small molecular ligands at the stage of structural intermediates. J. Struct. Biol. 130, 232–246.
D’Hooge R., Nagels G., Westland C. E., Mucke L., and De Deyn P. P. (1996) Spatial learning deficit in mice expressing human 751-amino acid beta-amyloid precursor protein. Neuroreport 7, 2807–2811.
Dodart J. C., Bales K. R., Gannon K. S., Greene S. J., DeMattos R. B., Mathis C., et al. (2002) Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat. Neurosci. 5, 452–457.
Hardy J. and Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356.
Harper J. D. and Lansbury P. T., Jr. (1997) Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66, 385–407.
Harper J. D., Wong S. S., Lieber C. M., and Lansbury P. T., Jr. (1999) Assembly of A beta amyloid protofibrils: an in vitro model for a possible early event in Alzheimer’s disease. Biochemistry 38, 8972–8980.
Hartley D. M., Walsh D. M., Ye C. P., Diehl T., Vasquez S., Vassilev P. M., et al. (1999) Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884.
Holcomb L., Gordon M. N., McGowan E., Yu X., Benkovic S., Jantzen P., et al. (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 4, 97–100.
Hsia A. Y., Masliah E., McConlogue L., Yu G. Q., Tatsuno G., Hu K., et al. (1999) Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA 96, 3228–3233.
Klein W. L., Krafft G. A., and Finch C. E. (2001) Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 24, 219–224.
Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., et al. (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 95, 6448–6453.
Lambert M. P., Viola K. L., Chromy B. A., Chang L., Morgan T. E., Yu J., et al. (2001) Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J. Neurochem. 79, 595–605.
Lorenzo A. and Yankner B. A. (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc. Natl. Acad. Sci. USA 91, 12,243–12,247.
McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., et al. (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 46, 860–866.
Moechars D., Dewachter I., Lorent K., Reverse D., Baekelandt V., Naidu A., et al. (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 274, 6483–6492.
Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., et al. (2000) High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058.
Nybo M., Svehag S. E., and Holm N. E. (1999) An ultra-structural study of amyloid intermediates in A beta1-42 fibrillogenesis. Scand. J. Immunol. 49, 219–223.
Podlisny M. B., Walsh D. M., Amarante P., Ostaszewski B. L., Stimson E. R., Maggio J. E., et al. (1998) Oligomerization of endogenous and synthetic amyloid beta-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry 37, 3602–3611.
Potempska A., Mack K., Mehta P., Kim K. S., and Miller D. L. (1999) Quantification of sub-femtomole amounts of Alzheimer amyloid beta peptides. Amyloid 6, 14–21.
Sian A. K., Frears E. R., El Agnaf O. M., Patel B. P., Manca M. F., Siligardi G., et al. (2000) Oligomerization of beta-amyloid of the Alzheimer’s and the Dutch-cerebral-haemorrhage types. Biochem. J. 349, 299–308.
Small G. W., Rabins P. V., Barry P. P., Buckholtz N. S., DeKosky S. T., Ferris S. H., et al. (1997) Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 278, 1363–1371.
Southwick P. C., Yamagata S. K., Echols C. L., Jr., Higson G. J., Neynaber S. A., Parson R. E., et al. (1996) Assessment of amyloid beta protein in cerebrospinal fluid as an aid in the diagnosis of Alzheimer’s disease. J. Neurochem. 66, 259–265.
Terry R. D., ed. (1999) The neuropathology of Alzheimer disease and the structural basis of its cognitive alterations, in Alzheimer Disease, Terry, Lippincott Williams & Wilkins, Philadelphia, PA, pp. 187–206.
Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., et al. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539.
Walsh D. M., Lomakin A., Benedek G. B., Condron M. M., and Teplow D. B. (1997) Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem. 272, 22,364–22,372.
Wang H. W., Pasternak J. F., Kuo H., Ristic H., Lambert M. P., Chromy B., et al. (2002) Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 924, 133–140.
Westerman M. A., Cooper-Blacketer D., Mariash A., Kotilinek L., Kawarabayashi T., Younkin L. H., et al. (2002) The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 22, 1858–1867.
Wolfe M. S. (2002) Secretase as a target for Alzheimer’s disease. Curr. Top. Med. Chem. 2, 371–383.
Yu J., Bakhos L., Chang L., Holterman M. J., Klein W. L., and Venton D. L. (2002) Per-6-substituted beta-cyclodextrin libraries inhibit formation of beta-amyloid-peptide (A beta)-derived, soluble oligomers. J. Mol. Neurosci. 19, 51–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, L., Bakhos, L., Wang, Z. et al. Femtomole immunodetection of synthetic and endogenous amyloid-β oligomers and its application to Alzheimer’s disease drug candidate screening. J Mol Neurosci 20, 305–313 (2003). https://doi.org/10.1385/JMN:20:3:305
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:20:3:305