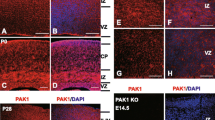
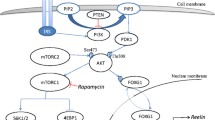
Abstract
Several of the genes currently known to be associated, when mutated, with mental retardation, code for molecules directly involved in Rho guanosine triphosphatase (GTPase) signaling. These include PAK3, a member of the PAK protein kinase family, which are important effectors of small GTPases. In many systems, PAK kinases play crucial roles regulating complex mechanisms such as cell migration, differentiation, or survival. Their precise functions in the central nervous system remain, however, unclear. Although their activity does not seem to be required for normal brain development, several recent studies point to a possible involvement in more subtle mechanisms such as neurite outgrowth, spine morphogenesis or synapse formation, and plasticity. This article reviews this information in the light of the current knowledge available on the molecular characteristics of the different members of this family and discuss the mechanisms through which they might contribute to cognitive functions.
Similar content being viewed by others
References
Bokoch G. M. (2003) Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781.
Chelly J. and Mandel J. L. (2001) Monogenic causes of X-linked mental retardation. Nat. Rev. Genet. 2, 669–680.
Sells M. A. (1999) Pictures in cell biology. Pak1 kinase activity affects the character of cell morphology and movement. Trends Cell. Biol. 9, 350–355.
Bienvenu T., des Portes V., McDonell N, et al. (2000) Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation. Am. J. Med. Genet. 93, 294–298.
Allen K. M., Gleeson J. G., Bagrodia S., et al. (1998) PAK3 mutation in nonsyndromic X-linked mental retardation. Nat. Genet. 20, 25–30.
Gedeon A. K., Nelson J., Gecz J., and Mulley J. C. (2003) X-linked mild non-syndromic mental retardation with neuropsychiatric problems and the missense mutation A365E in PAK3. Am. J. Med. Genet. A. 120, 509–517.
Hofmann C., Shepelev M., and Chernoff J., (2004) The genetics of Pak. J. Cell Sci. 117, 4343–4354.
Manser E., Chong C., Zhao Z. S., et al. (1995) Molecular cloning of a new member of the p21-Cdc42/Rac-activated kinase (PAK) family. J. Biol. Chem. 270, 25,070–25,078
Schmidt A. and Hall A. (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609.
Peck J., Douglas G., Wu C. H., and Burbelo P. D. (2002) Human RhoGAP domain-containing proteins: structure, function and evolutionary relationships. FEBS Lett. 528, 27–34.
Olofsson B. (1999) Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 11, 545–554.
Li Z., Hannigan M., Mo Z., et al. (2003) Directional sensing requires G βγ-mediated PAK1 and PIX α-dependent activation of Cdc42. Cell 114, 215–227.
Zhou G. L., Zhuo Y., King C. C., Fryer B. H., Bokoch G. M., and Field J. (2003) Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell Biol. 23, 8058–8069.
Yuan Z. Q., Kim D., Kaneko S., et al. (2005) ArgBP2gamma interacts with Akt and p21-activated kinase-1 and promotes cell survival. J. Biol. Chem. 280, 21,483–21,490.
Lei M., Lu W., Meng W., et al. (2000) Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102, 387–397.
Parrini M. C., Lei M., Harrison S. C., and Mayer B. J. (2002) Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol. Cell. 9, 73–83.
Jaffer Z. M., and Chernoff J., (2002) p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34, 713–717.
Frost J. A., Steen H., Shapiro P., et al., (1997) Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16, 6426–6438.
King A. J., Sun H., Diaz B., et al. (1998) The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396, 180–183.
Sundberg-Smith L. J., Doherty J. T., Mack C. P., and Taylor J. M. (2005) Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation. J. Biol. Chem. 280, 2055–2064.
Schmid R. S., Midkiff B. R., Kedar V. P., and Maness P. F. (2004) Adhesion molecule L1 stimulates neuronal migration through Vav2-Pak1 signaling. Neuroreport 15, 2791–2794.
Eblen S. T., Slack J. K., Weber M. J., and Catling A. D. (2002) Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell Biol. 22, 6023–6033.
Slack-Davis J. K., Eblen S. T., Zecevic M., et al. (2003) PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 162, 281–291.
Deroanne C, Vouret-Craviari V., Wang B., and Pouyssegur J. (2003) EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 116, 1367–1376.
Penzes P., Beeser A., Chernoff J., et al. (2003) Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron 37, 263–274.
Zhou H. and Kramer R. H. (2005) Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 280, 10,624–10,635.
Edwards D. C., Sanders L. C., Bokoch G. M., and Gill G. N. (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1, 253–259.
Gohla A. and Bokoch G. M. (2002) 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 12, 1704–1710.
Meng Y., Zhang Y., Tregoubov V., Falls D. L., and Jia Z. (2003) Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev. Neurosci. 14, 233–240.
Zhang H., Webb D. J., Asmussen H., Niu S., and Horwitz A. F. (2005) A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25, 3379–3388.
Vadlamudi R. K., Barnes C. J., Rayala S., et al. (2005) p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol. Cell Biol. 25, 3726–3736.
Diebold B. A., Fowler B., Lu J., Dinauer M. C., and Bokoch G. M. (2004) Antagonistic crosstalk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J. Biol. Chem. 279, 28,136–28,142.
Bryan B., Kumar V., Stafford L. J., Cai Y., Wu G., and Liu M. (2004) GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J. Biol. Chem. 279, 45,824–45,832.
Zenke F. T., Krendel M., DerMardirossian C., King C. C., Bohl B. P., and Bokoch G. M. (2004) p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J. Biol. Chem. 279, 18,392–18,400.
Hing H., Xiao J., Harden N., Lim L., and zipursky S. L. (1999) Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell 97, 853–863.
Ang, L. H., Kim J., Stepensky V., and Hing H. (2003) Dock and Pak regulate olfactory axon pathfinding in Drosophila. Development 130, 1307–1316.
Fan X., Labrador J. P., Hing H., and Bashaw G. J. (2003) Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron 40, 113–127.
Parnas D., Haghighi A. P., Fetter R. D., Kim S. W., and Goodman C. S. (2001) Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron 32, 415–424.
Melzig J., Rein K. H., Schafer U., et al. (1998) A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr. Biol. 8, 1223–1226.
Schneeberger D. and Raabe T. (2003) Mbt, a Drosophila PAK protein, combines with Cdc42 to regulate photoreceptor cell morphogenesis. Development 130, 427–437.
Udo H., Jin I., Kim J. H., et al. (2005) Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron 45, 887–901.
Sells M. A., Boyd J. T., and Chernoff J. (1999) p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 145, 837–849.
Dharmawardhane S., Sanders L. C., Martin S. S., Daniels R. H., and Bokoch G. M. (1997) Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J. Cell Biol. 138, 1265–1278.
Burbelo P. D., Kozak C. A., Finegold A. A., Hall A., and Pirone D. M. (1999) Cloning, central nervous system expression and chromosomal mapping of the mouse PAK-1 and PAK-3 genes. Gene 232, 209–215.
Daniels R. H., Hall P. S., and Bokoch G. M. (1998) Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J 17, 754–764.
Rashid T., Banerjee M., and Nikolic M. (2001) Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology. J. Biol. Chem. 276, 49,043–49,052.
Shekarabi M., Moore S. W., Tritsch N. X., Morris S. J., Bouchard J. F., and Kennedy T. E. (2005) Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 25, 3132–3141.
Hayashi M. L., Choi S. Y., Rao B. S., et al. (2004) Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron 42, 773–787.
Ramakers G. J. (2002) Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 25, 191–199.
Ropers H. H. and Hamel B. C. (2005) X-linked mental retardation. Nat. Rev. Genet. 6, 46–57.
Matus A. (2000) Actin-based plasticity in dendritic spines. Science 290, 754–758.
Harris K. M. (1999) Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 9, 343–348.
Yuste R. and Bonhoeffer T. (2004) Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 5, 24–34.
Luscher C., Nicoll R. A., Malenka R. C., and Muller D. (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 3, 545–550.
Malinow R. and Malenka R. C. (2002) AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126.
Kohn M., Steinbach P., Hameister H., and Kehrer-Sawatzki H. (2004) A comparative expression analysis of four MRX genes regulating intracellular signalling via small GTPases. Eur. J. Hum. Genet. 12, 29–37.
Wilda M., Bachner D., Zechner U., Kehrer-Sawatzki H., Vogel W., and Hameister H. (2000) Do the constraints of human speciation cause expression of the same set of genes in brain, testis, and placenta? Cytogenet. Cell Genet. 91, 300–302.
Obermeier A., Ahmed S., Manser E., Yen S. C., Hall C., and Lim L. (1998) PAK promotes morphological changes by acting upstream of Rac. EMBO J. 17, 4328–4339.
Boda B., Alberi S., Nikonenko I., et al. (2004) The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J. Neurosci. 24, 10,816–10,825.
Purpura D. P. (1974) Dendritic spine “dysgenesis” and mental retardation. Science 186, 1126–1128.
Meng J., Meng Y., Hanna A., Janus C., and Jia Z. (2005) Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J. Neurosci. 25, 6641–6650.
McPhie D. L., Coopersmith R., Hines-Peralta A., et al. (2003) DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. J. Neurosci. 23, 6914–6927.
Jakobi R., McCarthy C. C., Koeppel M. A., and Stringer D. K. (2003) Caspase-activated PAK-2 is regulated by subcellular targeting and proteasomal degradation. J. Biol. Chem. 278, 38,675–38,685.
Nunn M. F. and Marsh J. W. (1996) Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 70, 6157–6161.
Shin E. Y., Shin K. S., Lee C. S., et al., (2002) Phosphorylation of p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange factor, via the Ras/ERK/PAK2 pathway is required for basic fibroblast growth factor-induced neurite outgrowth. J. Biol. Chem. 277, 44,417–44,430.
Abo A., Qu J., Cammarano M. S., et al. (1998) PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17, 6527–6540.
Dan C., Kelly A., Bernard O., and Minden A. (2001) Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J. Biol. Chem. 276, 32,115–32,121.
Callow M. G., Clairvoyant F., Zhu S., et al. (2002) Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277, 550–558.
Zhang H., Li Z., Viklund E. K., and Stromblad S. (2002) P21-activated kinase 4 interacts with integrin alpha v beta 5 and regulates alpha v beta 5-mediated cell migration. J. Cell Biol. 158, 1287–1297.
Qu J., Li X., Novitch B. G., et al. (2003) PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol. Cell Biol. 23, 7122–7133.
Cau J., Faure S., Comps M., Delsert C., and Morin N. (2001) A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. J. Cell Biol. 155, 1029–1042.
Dan C., Nath N., Liberto M., and Minden A. (2002) PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol. Cell Biol. 22, 567–577.
Pandey A., Dan I., Kristiansen T. Z., et al. (2002) Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 21, 3939–3948.
Cotteret S., Jaffer Z. M., Beeser A., and Chernoff J. (2003) p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol. Cell Biol. 23, 5526–5539.
Li X. and Minden A. (2003) Targeted disruption of the gene for the PAK5 kinase in mice. Mol. Cell Biol. 23, 7134–7142.
Yang F., Li X., Sharma M., Zarnegar M., Lim B., and Sun Z. (2001) Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 276, 15,345–15,353.
Lee S. R., Ramos S. M., Ko A., et al. (2002) AR and ER interaction with a p21-activated kinase (PAK6). Mol. Endocrinol. 16, 85–99.
Duboule D. and Wilkins A. S. (1998) The evolution of bricolage. Trends Genet. 14, 54–59.
Luo L. (2000) Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173–180.
Lisman J. (2003) Actin's actions in LTP-induced synapse growth. Neuron 38, 361–362.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boda, B., Nikonenko, I., Alberi, S. et al. Central nervous system functions of PAK protein family. Mol Neurobiol 34, 67–80 (2006). https://doi.org/10.1385/MN:34:1:67
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:34:1:67