Involvement of HCN Channel in Muscarinic Inhibitory Action on Tonic Firing of Dorsolateral Striatal Cholinergic Interneurons
- State Key Laboratory of Toxicology and Medical Countermeasures, Department of Biochemical Pharmacology, Beijing Institute of Pharmacology and Toxicology, Beijing, China
The striatum is the most prominent nucleus in the basal ganglia and plays an important role in motor movement regulation. The cholinergic interneurons (ChIs) in striatum are involved in the motion regulation by releasing acetylcholine (ACh) and modulating the output of striatal projection neurons. Here, we report that muscarinic ACh receptor (M receptor) agonists, ACh and Oxotremorine (OXO-M), decreased the firing frequency of ChIs by blocking the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Scopolamine (SCO), a nonselective antagonist of M receptors, abolished the inhibition. OXO-M exerted its function by activating the Gi/o cAMP signaling cascade. The single-cell reverse transcription polymerase chain reaction (scRT-PCR) revealed that all the five subtypes of M receptors and four subtypes of HCN channels were expressed on ChIs. Among them, M2 receptors and HCN2 channels were the most dominant ones and expressed in every single studied cholinergic interneuron (ChI).Our results suggest that ACh regulates not only the output of striatal projection neurons, but also the firing activity of ChIs themselves by activating presynaptic M receptors in the dorsal striatum. The activation of M2 receptors and blockage of HCN2 channels may play an important role in ACh inhibition on the excitability of ChIs. This finding adds a new G-protein coupled receptor mediated regulation on ChIs and provides a cellular mechanism for control of cholinergic activity and ACh release in the dorsal striatum.
Introduction
As a prominent nucleus in the basal ganglia, striatum serves as a center of input and integration for cortical, thalamic, and midbrain afferents. The striatum is functionally divided into two parts, along a dorsolateral/ventromedial axis, which exert different roles in cognitive, affective, and limbic functions (Smith and Kieval, 2000; Voorn et al., 2004). The striatum is composed of projection neurons, cholinergic interneurons (ChIs), and other GABAergic interneurons (Vincent et al., 1983; Chesselet and Graybiel, 1986; Smith and Parent, 1986; Cowan et al., 1990; Bennett and Bolam, 1993).
ChIs only take a small fraction of striatal neurons (1–3%), but have widespread connections throughout the striatum (Kawaguchi et al., 1995; Tepper and Bolam, 2004). They synthesize, transport, and secrete acetylcholine (ACh; Woolf and Butcher, 1981; Wang et al., 2006; Ding et al., 2010; Goldberg et al., 2012). Despite their small numbers, these giant and spiny ChIs are responsible for striatal levels of ACh, which is among the highest in the brain (Mesulam et al., 1992; Contant et al., 1996). Increased release of ACh by ChIs has been shown to contribute to structural changes and distorted network function in the striatum (Pisani et al., 2007).
ChIs have been proposed to regulate the duration, strength, and spatial pattern of action potentials in striatal local circuits (Galarraga et al., 1999; Calabresi et al., 2000; Koós and Tepper, 2002). Their dysfunctions are involved in behavior and other movement disorders such as Parkinson’s disease (Apicella et al., 1997; Blazquez et al., 2002; Morris et al., 2004; Joshua et al., 2008; Witten et al., 2010). By activating muscarinic receptors (M receptors), ACh exerts its profound modulatory effect on postsynaptic neurons. M receptors are divided into two classes: M1-class (M1, M3, and M5) and M2-class (M2, M4). M1-class receptors couple to Gαq proteins that activate phospholipase C (PLC) signal cascade. M2-class receptors preferentially couple to Gαi proteins that inhibit adenylyl cyclase (AC) and downregulate intracellular cAMP content. M receptors have been reported to be widely expressed in the striatum, and all five M receptor subtypes (M1–5) are expressed in the dorsal striatum (Eglen, 2012). M1-class receptors are mostly distributed on the postsynaptic membrane, while M4 receptors are restricted to the striatonigral medium-size spiny neurons (MSNs) and neuropeptide-Y releasing interneurons (Ince et al., 1997; Yan et al., 2001). M2 receptors are considered to be mainly expressed in ChIs (Weiner et al., 1990; Bernard et al., 1992) where they function as cholinergic autoreceptors and regulate ACh release (Alcantara et al., 2001). However, M4 receptors are also reported to be expressed on ChIs as autoreceptors (Bernard et al., 1992; Hersch et al., 1994; Yan and Surmeier, 1996).
ChIs are pacemaking neurons and present distinct burst-pause patterns in their tonic firings during motor learning and reward-related behaviors. Autonomous pacemaking activity in ChIs is mainly driven by the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Bennett and Wilson, 1999; Wilson, 2005). The HCN currents (Ih) exert regulatory effects on intrinsic ChIs’ excitability by depolarizing the membrane to its firing threshold of action potentials (Bennett et al., 2000; Wilson, 2005).
In mammals, the HCN channels are encoded by four genes (HCN1-4), which present various electrophysiological properties, activation kinetics, and cAMP-sensitivity, and are widely expressed throughout the heart and the central nervous system (Santoro et al., 2000; Ulens and Siegelbaum, 2003; Biel et al., 2009). Ih is considered to be involved in at least four physiological processes: “(1) control of pacemaker activity; (2) control and limitation of resting potential; (3) control of membrane resistance and dendritic integration; and (4) regulation of synaptic transmission” (Robinson and Siegelbaum, 2003). All four HCN isoforms are expressed in the brain (Biel et al., 2009). HCN expression patterns have been characterized in some nucleus, such as hippocampus. However, the identification of HCN channel subunits expressed in ChIs is unclear.
It has been confirmed that dopamine modulates the pause response in tonic firing in ChIs by inhibiting HCN channels (Deng et al., 2007). And there are also reports that ACh can regulate the ChIs’ activity through voltage- and/or ligand-gated channels (Yan and Surmeier, 1996; Calabresi et al., 1998; Pisani et al., 1999; Ding et al., 2006, 2010; Bonsi et al., 2008). However, whether HCN channels are directly involved in the muscarinic modulation on the excitability of ChIs has not been reported. We speculate that application of M receptor agonist could activate M receptors expressed on ChIs and thus downregulate internal cAMP, which would result in the reduction of Ih and inhibition of spontaneous firing.
To test our hypothesis, we first used single cell RT-PCR with subtype-specific primers to identify the distribution of HCN and M receptor subtypes. Then, we observed the effect of M receptor agonists on Ih and firing activity of ChIs. Our data reveal that all the HCN subunits are expressed on ChIs but HCN2 is the most abundant one. In terms of M receptors, all four subtypes are found in some ChIs but M2 is predominantly expressed. Furthermore, application of M receptor agonist depresses the firing activity of ChIs. Therefore, we propose that the activation of M2 receptors and blockage of HCN2 channels underlie the ACh inhibition on the excitability of ChIs.
Materials and Methods
Brain Slice Preparation
All experiments were approved by the Animal Research Advisory Committee of Beijing Institute of Biological Science and in accordance with the NIH guideline (Publication No. 85–23, revised 1985) to the care and use of laboratory animals. Preparation of striatal slices was carried out similar to those previously described (Bennett and Wilson, 1999; Deng et al., 2005; Sciamanna et al., 2011). Briefly, male Sprague Dawley rats (14–16 days old) were killed by cervical dislocation. The brain was quickly removed from the skull and submerged in ice-cold (4°C) oxygenated sucrose solution containing (in mM): 230 sucrose, 2.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 10 glucose, 10 MgSO4, 0.5 CaCl2, 2 sodium pyrurate, and adjusted pH 7.4 with NaOH, 295–305 mOsm/L. Coronal striatal slices (300 μm) were cut using a vibratome (MA752, Campden instruments). Slices, put in a chamber (Warner instruments) bubbled with a 95% O2 and 5% CO2 gas mixture, were incubated in the standard NaHCO3-buffered saline solution containing (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 2 sodium pyrurate, 2 CaCl2, 2 MgCl2, pH 7.4 with HCl (300–305 mOsm/l), for 30 min at 32°C. The chamber was then maintained at room temperature continuously bubbled with O2/CO2 gas mixture. The slices could be stored in vitro for several hours while maintaining excellent viability prior to electrophysiological experiment.
Electrophysiological Recording
A single slice was transferred to the recording chamber and submerged in a continuously flowing NaHCO3-buffered saline (1.5–2 ml/min) bubbled with a 95% O2 and 5% CO2 gas mixture at room temperature (~25°C). Recording electrodes were prepared from borosilicate glass (Sutter instruments, Novato, CA, USA) using a horizontal electrode puller (P-97, Sutter instruments, Novato, CA, USA). The electrodes had resistance of 2–4 MΩ when filled with the internal solution consisted of (in mM): 130 K+-gluconate, 10 HEPES, 10 KCl, 5 EGTA, 1 CaCl2, 1 MgCl2, 2 Na2ATP, 0.5 Na3GTP, pH 7.4, 295–300 mOsm/L. The slice was visualized with a 40× water-immersion objective (NIR Apo, Nikon, Japan) using standard infrared and differential interference contrast (IR-DIC) microscopy and a CCD camera. Cells in the dorsolateral striatum up to ~50 μm beneath the slice surface were patched and monitored. Recording in normal current-clamp or voltage-clamp mode was performed with an Axon 200B amplifier (Molecular devices, Foster city, CA, USA) and Clampex 10.1 software (Molecular devices) at room temperature (~25°C; Bennett and Wilson, 1999; Nolan et al., 2003; Hawkins et al., 2015). After tight-seal (>1 GΩ) formation, fast and slow capacitance compensation was performed. During the whole-cell recording, series resistance was compensated (80–90%) and monitored periodically. Neurons were excluded from the analysis when their series resistance was above 50 MΩ or changed by more than 25% during the experiment. Data were filtered at 2 kHz and acquired at sampling rate of 10 kHz.
Modified NaHCO3-buffered saline for recording Ih had the composition (mM): 115 NaCl, 5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 glucose, 2 sodium pyrurate, 2 CaCl2, 2 MgCl2, pH 7.4. BaCl2 (1 mM) and TTX (0.5 μM) were added to the saline to block inward rectifier K+ and Na+ channels, respectively. CdCl2 (0.1 mM), 4-aminopyridine (2 mM), and tetraethylammonium (5 mM) were also added to saline to block voltage-dependent Ca2+ and K+ channels, respectively (Nolan et al., 2003; Deng et al., 2007). In the present study, synaptic blockers were not used because the ChIs receive minimal synaptic inputs in vitro, and these inputs have an undetectable effect on the spontaneous firing rates and patterns exhibited by these cells (Bennett and Wilson, 1999). Ih was activated using hyperpolarizing voltage steps from a holding potential of −50 mV to −140 mV in 10 mV decrements in voltage-clamp model. To ensure the stability of whole-cell recordings, the sweep start-to-start interval was 5 s. The amplitude of Ih was calculated by subtracting the current value at the onset of hyperpolarizing voltage (peak amplitude) from that at the end (mean current of the 20 ms before the termination of each voltage step; Kodirov et al., 2014).
Histochemical Staining for Biocytin-Loaded Slices
To identify the morphology of recorded cells, 0.2% (w/v) biocytin was added into the pipette solution to stain cells by diffusion (Horikawa and Armstrong, 1988). After termination of recording, the slice containing the cell injected with biocytin was fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 at 4°C for 10 h and then incubated in PBS containing 1% Triton x-100 (TX) for 2 h at room temperature. The slices were then washed with 0.01 M PBS three times for 10 min each time. And then the slices were incubated in PBS containing 3% H2O2 for 10 min to suppress endogenous peroxidase activity and washed in PBS three times for total of 15 min. They were then incubated in PBS containing avidin-biotin-peroxidase complex (ABC solution, Fuzhou Maixin company, China) for 10 min. 0.05% (w/v) 3,3′-Diaminobenzidine tetrahydrochloride (DAB) 0.1–0.2 ml was dropped on the slices to react for 3–5 min, then washed in PBS three times for 5 min each.
To ensure the biocytin-loaded cells were ChIs, the biocytin-loaded slices were further processed with immunofluorescence histochemical staining. The slices were blocked using rabbit serum at room temperature for 1 h and then incubated with a goat polyclonal antibody against choline acetyltransferase (ChAT; Millipore, Cat# AB144P, RRID: AB_2079751 using at 1:100) in PBS overnight at 4°C. After 3 × 5 min washing in PBS, the slices were incubated in rabbit serum containing the Rhodamine (TRITC)-conjugated affinipure rabbit anti-goat IgG (1:200) for 1 h at room temperature. After 3 × 5 min washing in PBS once more, the slices were glycerol-mounted on slides and photographed under a fluorescence microscope (BX51 Olympus optical, Japan).
Histochemical Staining for Perfusion-Fixed Brains
For histochemical experiments, animals of similar age to those used for electrophysiological experiments were used. The animals were deeply anesthetized with overdose of Nembutal, and perfused transcardially with phosphate buffered saline first, followed by 200 ml ice-cold fixative containing 4% paraformaldehyde in PBS, pH7.4. The brain was removed carefully, cut sagittally at the midline and post-fixed in the same fixative for 3 h at 4°C. After incubation in PBS containing 30% (w/v) sucrose for 3 d at 4°C, the brain was sectioned at 12 μm thickness, and slices containing the striatum were mounted on polylysine-coated slides. Four primary antibodies were ordered from commercial companies: a rabbit anti-HCN 1 antibody (Alomone Labs, Cat# APC-056, RRID: AB_2039900 using at 1:100), a rabbit anti-HCN 2 antibody (Alomone Labs, Cat# APC-030, RRID: AB_2313726 using at 1:100), a rabbit anti-M2 receptor antibody (Abcam, Cat# ab109226, RRID: AB_10858602 using at 1:200), and a mouse anti-M4 receptor antibody (Abcam, Cat# ab77956, RRID: AB_1566454 using at 1:200).
Sections were permeablized in 0.1% TX in PBS for 1 h, blocked with normal serum, which was from the same host of secondary antibody, for 1 h, and incubated overnight at 4°C with the primary antibody diluted in PBS containing 1% BSA. Then, sections were rinsed with PBS for 3 × 5 min and transferred to secondary antibody in blocking serum for 1 h at room temperature in dark. After another three washes, the sections were mounted in medium fluoroshieldTM with DAPI (Sigma-aldrich F6057, Saint Louis, MO, USA) and coverslipped. Then they were subsequently photographed using a confocal laser scanning microscope (Zeiss, LSM510). The control experiment for immunohistochemical labeling specificity of the secondary antibody includes the omission of the primary antibody and the use of preimmune normal serum (Tozzi et al., 2011). The result indicated that there was no nonspecific labeling of neural soma or processes.
Single-Cell Reverse Transcription-Polymerase Chain Reaction (scRT-PCR)
HCN channel and M receptor mRNAs in striatal ChIs were detected by using techniques similar to those described previously (Surmeier et al., 1996; Yan and Surmeier, 1996; Tkatch et al., 2000). Neurons were subjected to whole cell voltage-clamp recording before aspiration. To maximize mRNA yields, some neurons were aspirated without recording with an electrode containing ~4 μl of sterile water. Neurons were aspirated into the patch electrode by applying negative pressure. After aspiration, the electrode was removed from the holder, the tip was broken, and the content was ejected into a 0.5 ml eppendorf tube containing 0.5 μl of oligo-dT, 0.5 μl of random primer, 0.25 μl of RNasin (40U/μl). The primer mixture was heated to 70°C for 5 min and then immediately chilled in ice water for at least 5 min. The reverse transcription (RT) reaction mixture, total of approximately ~20 μl, was composed of the pipette tip content, primer mixture, GoScriptTM 5× Reaction buffer (4 μl), MgCl2 (2.4 μl), dNTP (1 μl), RNasin (0.25 μl), GoScriptTM Reverse Transcriptase (1.0 μl), and nuclease-free water (6.35 μl). Single-strand cDNA was synthesized following this step: annealing at 25°C for 5 min, extending at 42°C for 60 min, inactivating reverse transcriptase at 70°C for 15 min, and then icing. The RNA strand in the RNA-DNA hybrid was removed by adding 1 μl of RNase H (2 U/μl) and incubating for 20 min at 37°C. All reagents were obtained from Promega Inc (Madison, WI, USA). All the semi-quantitative experiments presented here were conducted with the same enzyme lot.
The cDNA from the RT in a single striatal neuron was amplified using PCR protocols modified from Surmeier’s report (Surmeier et al., 1996). Amplification was performed with one of the two protocols. The conventional one-stage amplification protocol was carried out in a thermal cycler (Applied Biosystems) with thin-walled plastic tubes. Detection experiments were carried out using one-tenth of the single-cell cDNA (2μl) as a template for the PCR. Reaction mixture contained 2–2.5 mM MgCl2, 0.2 mM each dNTP, 0.8 μM primers, 1.25U GoTaq G2 flexi DNA polymerase, and 10 μl green GoTaq flexi buffer. Nuclease-free water was added to final volume of 50 μl. The thermal cycling program for all reactions was set in three steps: step1, 94°C for 3 min; step2, for 42 cycles, 94°C for 1 min, 58°C for 1 min, 72°C for 1 min; and step 3, 72°C for 5 min.
In the semi-quantitative RT-PCR experiment, the modified two-stage amplification protocol was designed to maximize our ability to detect low abundance of mRNAs of HCN channels or M receptors (Yan and Surmeier, 1996). In the first stage, 2μl cDNA from RT was used as template (Surmeier et al., 1996; Yan and Surmeier, 1996; Tkatch et al., 2000). All HCN channel or M receptor primers were added to a reaction mixture containing the same concentration of reagents as in the conventional one-stage amplification protocol, except for slightly elevated MgCl2 (3.5~4.0 mM) and dNTPs (1.0 mM; Chamberlain and Chamberlain, 1994). The same program as the conventional one-stage amplification protocol was performed in 13 cycles. In the second stage, an aliquot (1/10) of the first stage PCR product (5μl) was serially diluted and used as a template for a second round of “touch down” PCR amplification with each pair of specific primers. Thirty two cycles were performed with the same program as the first stage.
The PCR primers were synthesized either by the Beijing Bomaide or Beijing Huada Inc. PCR primers for glutamate decarboxylase 67 (GAD67), β-actin, GAPDH, ChAT, HCN channels, and M receptors were described previously (Yan and Surmeier, 1996; Tkatch et al., 1998; Budde et al., 2005). PCR procedures were performed using procedures designed to minimize the chance of cross-contamination (Cimino et al., 1990). Negative controls for contamination from extraneous and genomic DNAs were run for every batch of neurons. To ensure that genomic DNA did not contribute to the PCR products, neurons were aspirated and processed in the normal manner, except that the reverse transcriptase was omitted. Contamination from extraneous DNA was checked by replacing the cellular template with water. Both controls were consistently negative in these experiments.
PCR products were visualized by staining with ethidium bromide and analyzed by the electrophoresis in 2% agarose gels. In representative cases, amplicons were purified from the gel, and products were sequenced and verified in Beijing Bomaide or Beijing Huada Inc.
Drug Application and Data Analysis
All drugs were purchased from Sigma-Aldrich (St. Louis, MI, USA) except noted specially. Drugs were dissolved as concentrated stocks in either water or DMSO and stored at −20°C. When DMSO stock solution was used, equivalent amounts of DMSO were added to buffer as controls, and the final concentration of DMSO should not exceed 0.1%. Working solutions with different drugs were prepared just before use. During experiments, drugs, except otherwise marked, were applied in the flowing bath solutions. GDP-β-S was added into the intracellular solution in recording pipette. Total replacement of the medium in the recording chamber occurred within 1 min.
Data analysis was performed with software including Clampfit Version 10.2, Prism Version 6.0, and Origin Version 9.0. The relative Ih, as shown in Figures 2, 7, was determined as B/A*100%, in which A and B represents the Ih recorded before and after the application of drugs, at the −140 mV hyperpolarizing voltage, separately. All results were presented as mean ± SD. Statistical analysis was performed using student’s t-test (paired where relevant), the one-way ANOVA, and the two-way ANOVA. Difference of p < 0.05 was considered statistically significant. The threshold probability of single cell PCR detection was fitted by the Gaussian curve regression.
Results
Identification of ChIs in Dorsolateral Striatum
To guarantee the neurons we studied are ChIs, we identified them based on their morphological, electrophysiological, and histochemical features (Paxinos and Watson, 1986; Kawaguchi, 1993; Bennett and Wilson, 1998). As shown in Figure 1A, neurons under IR-DIC visualization with large soma and thick primary dendrites were initially targeted. In scRT-PCR experiments, the cells selected preferentially in morphology transcribed ChAT mRNA while GAD67 products were not detected (Figure 1B), which could exclude the contamination of MSNs. Examination of biocytin-filled neurons revealed that the thick primary dendrites branched further to form secondary and higher order smaller-diameter dendrites (Figure 1C). In several cases, the identity of the recording neuron was further verified through a histochemical staining (Figure 1D). Every neuron loaded with biocytin also expressed ChAT (Figure 1E).
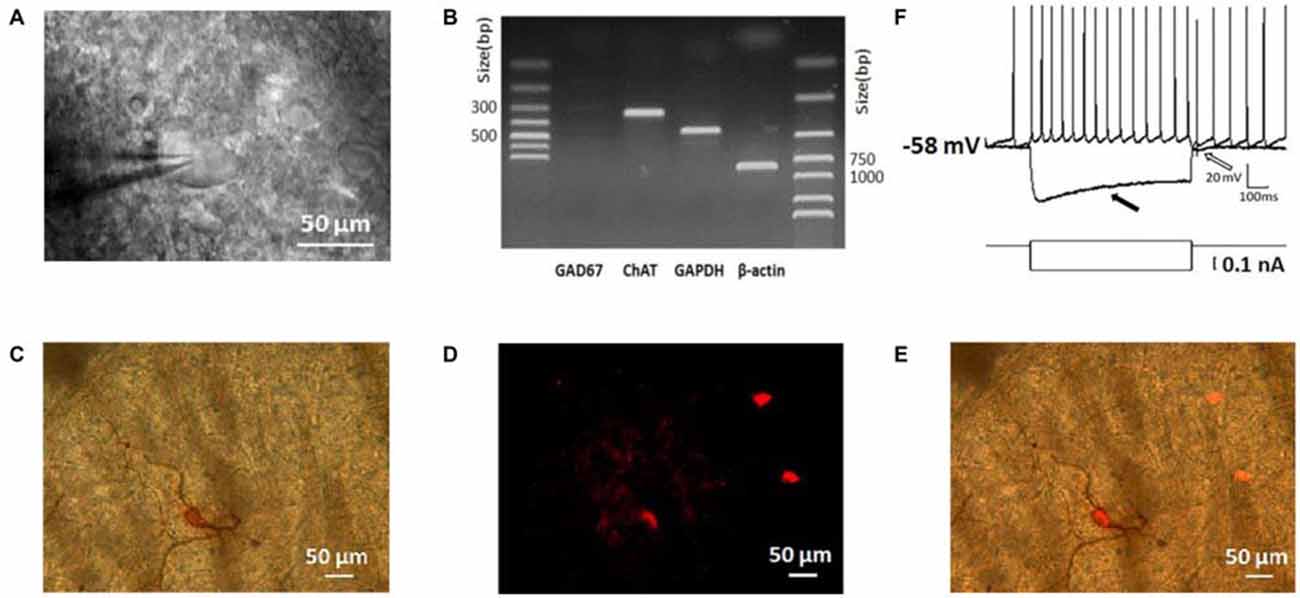
Figure 1. Morphological, physiological, electrophysiological, and immunohistochemical staining characterization of striatal cholinergic interneurons (ChIs). (A) An IR-DIC image of a dorsolateral striatal slice illustrating the characteristic appearance of giant interneurons. (B) ChAT was shown in single-cell reverse transcription polymerase chain reaction (scRT-PCR) experiment product. The absence of GAD 67 indicated aspirated cellular content wasn’t contaminated by MSNs. (C) Micrograph of a giant cell loaded with biocytin and subsequently stained immunohistochemically. (D) Immunofluorescence staining of ChIs in biocytin-loaded slices. (E) Merge of (C) and (D). The targeted cell intracellular loaded biocytin was co-stained by ChAT, inferring the giant aspiny ChIs. (F), Depolarizing somatic current injection elicited a train of regular spiking followed by a large hyperpolarization (indicated by a hollow arrow). Negative current injection caused a large hyperpolarization followed by a sag (indicated by a filled arrow).
Depolarization of the membrane potential elicited repetitive firings in recorded neurons that were followed by a large-amplitude and long duration of after-hyperpolarization (AHP; indicated by a hollow arrow in Figure 1F). On the other hand, an initial hyperpolarization was followed by a subsequent sag (indicated by a solid arrow in Figure 1F), indicating the presence of a cation current, Ih presumably. In slices, the majority of neurons was tonically active and showed spontaneous activity. Moreover, the recorded neurons present electrophysiological characters that are consistent with that of the striatal ChIs described previously (Bennett and Wilson, 1999; Sanchez et al., 2011; Ponterio et al., 2013).
Characterization of Ih in ChIs
As Deng et al. (2007) reported, we isolated Ih from ChIs with the modified NaHCO3-buffered saline described in methods section. Time- and voltage- dependent inward currents were activated in a voltage-clamp mode by a series of hyperpolarizing pulses from −50 mV to −140 mV in a 10 mV decrement (Figure 2A). To confirm the inward currents are Ih, HCN channel blocker ZD7288 and Cs+ were used. When ZD7288 or Cs+ were applied to the perfusion, the hyperpolarized sag currents were remarkably depressed (Figure 2B), which confirmed the Ih identification. As shown in Figure 2C, at the −140 mV hyperpolarizing voltage, application of ZD7288 (30 μM) dramatically blocked the Ih by 94.50% ± 0.99% (n = 7), and the presence of CsCl (1 mM) inhibited the Ih by 90.74% ± 1.13% (n = 5) respectively.
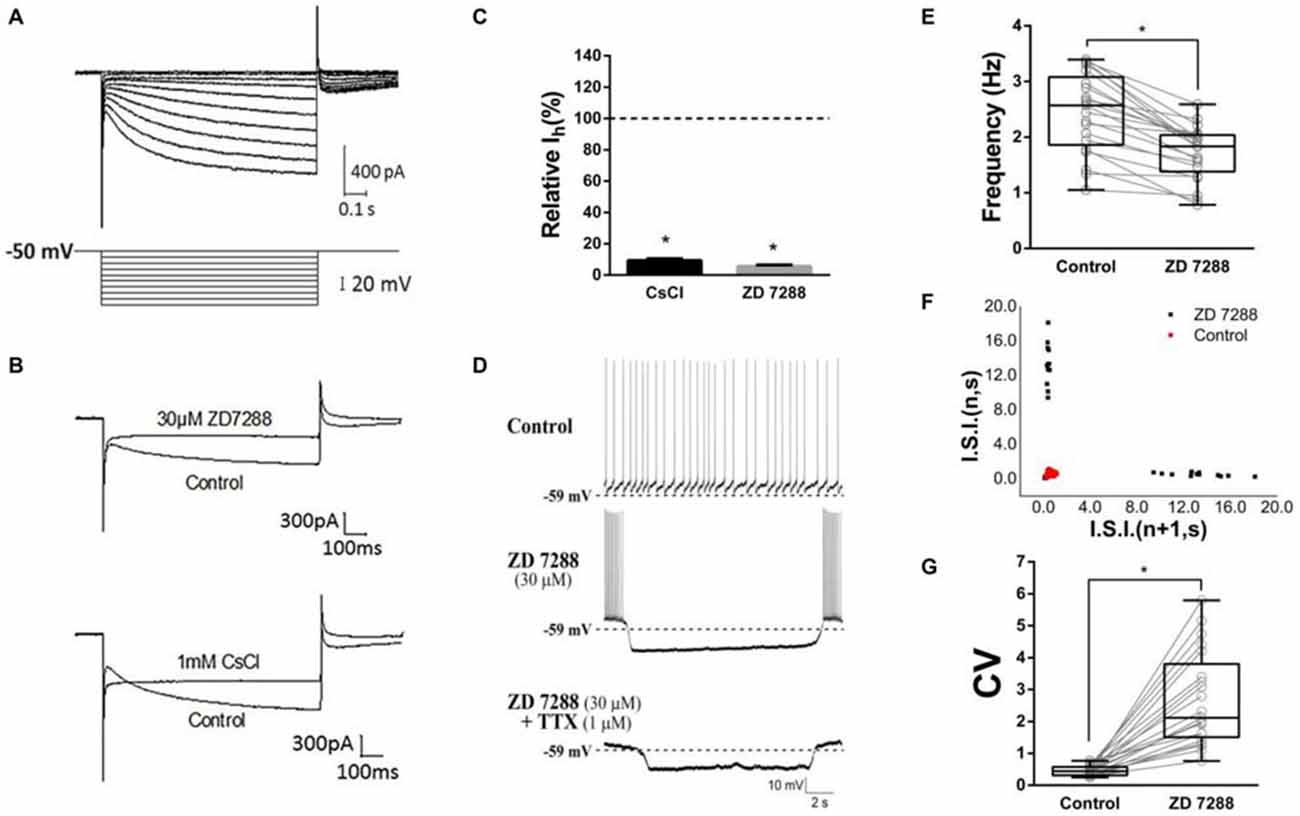
Figure 2. Contribution of HCN currents (Ih) to tonic firing. (A) The specific Ih was isolated in dorsolateral striatal ChIs. (B) Example traces of Current evoked at the −140 mV hyperpolarizing voltage before and after bath application of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel blockers, ZD7288 (30 μM) or Cs+ (1 mM). (C) ZD7288 and CsCl significantly blocked the Ih. (D) ZD7288 (30 μM) induced a discontinuously hyperpolarized pause and altered the spiking pattern. The induced action potential bursts were block by TTX but not the subthreshold membrane potential oscillation. (E) ZD7288 significantly decreased the overall event frequency of spiking (*p < 0.001, paired student’s t-test, n = 21). (F) A representative scatter of joint consecutive interspike interval (I.S.I.) plot of the discharge. (G) ZD7288 significantly increased the coefficient of variation (CV) of the I.S.I frequency (*p < 0.001, paired student’s t-test, n = 21).
To test whether Ih plays a role in determining the firing pattern of ChIs, the effect of ZD7288 was observed in standard NaHCO3-buffered saline. We found that application of ZD7288 (30 μM) induced a discontinuously hyperpolarized pause and changed the spiking pattern into oscillation with intra-bursting frequency of 6.21 ± 2.18 Hz (middle panel in Figure 2D, n = 21). Application of TTX only abolished the burst spiking but did not change the effects of subthreshold membrane potential oscillation (lower panel in Figure 2D, n = 21). In presence of ZD7288, the overall event frequency of spiking was reduced from 2.44 ± 0.71 Hz to 1.72 ± 0.48 Hz (Figure 2E, n = 21, p < 0.001). The coefficient of variation (CV) of joint consecutive inter-spike interval (I.S.I.) was used to describe the irregularity of pacemaking rhythm in ChIs. Application of ZD7288 altered CV value significantly from 0.46 ± 0.16 of the control group to 2.67 ± 1.46 (Figures 2F,G, n = 21, p < 0.001). These results indicated that Ih is critical to the firing pattern of ChIs.
HCN Channels Expression on ChIs
In the striatum, the ChIs express HCN channels and characteristically have a pronounced hyperpolarization-activated sag potential (Kawaguchi, 1993; Bennett et al., 2000). To investigate the HCN channel identity expressed on ChIs, we carried out scRT-PCR experiment by sucking the cellular content into the recording pipette while avoiding its nucleus during the procedure. As shown in Figure 3A, the cell with large soma was aspirated, leaving a swollen nucleus in the original position. The cellular content of ChI was used for the conventional one-stage PCR amplification protocol. A representative PCR result was shown in Figure 3B. In this neuron, PCR products, separated by the electrophoresis in 2% agarose gels, stained with ethidium bromide and visualized by UV light, indicated that four isoforms of HCN channels were detected. Furthermore, HCN2 and HCN3 are more abundant compared with HCN4 and HCN1. The statistical results of 27 analyzed cells indicated that mRNAs of HCN2 and HCN3 were found in every neuron, whereas mRNAs of HCN1 and HCN4 were found in partial neurons with ratio of 11/27 and 26/27, respectively (Figure 3C).
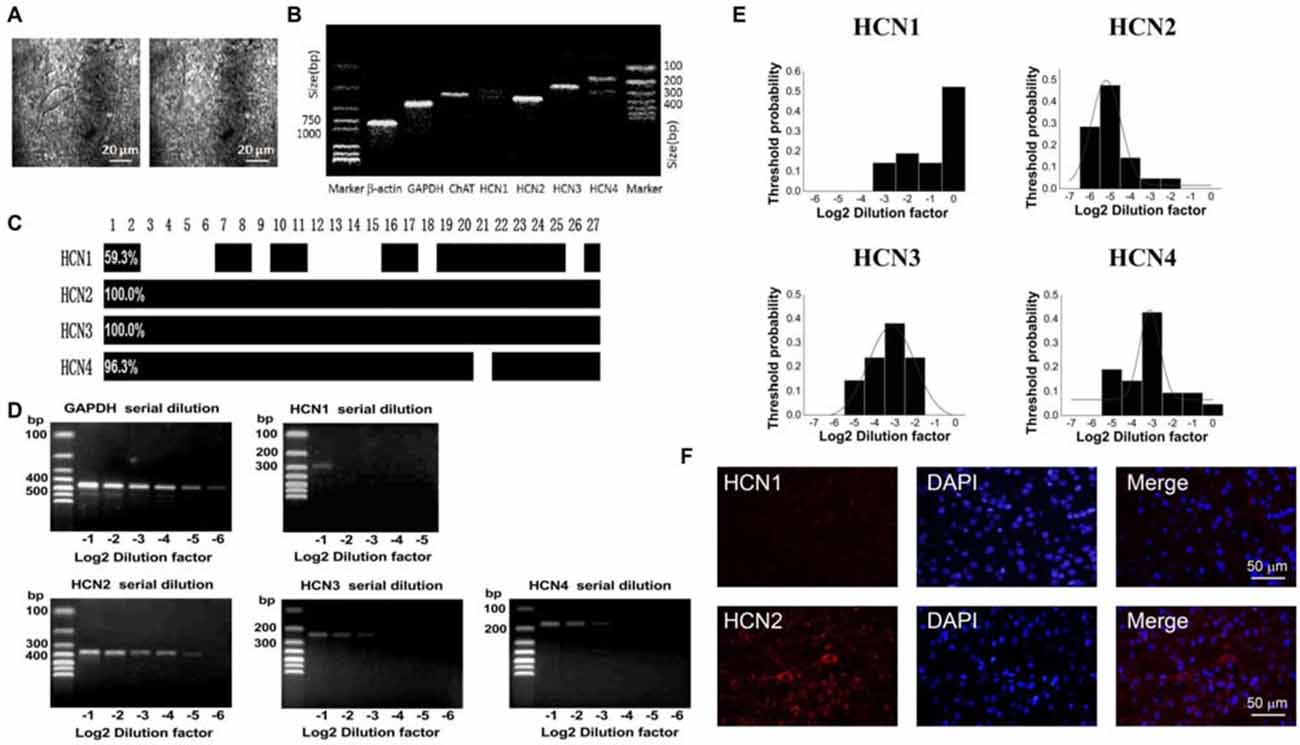
Figure 3. HCN2 channel was dominantly expressed on ChIs. (A) Schematic graph showed the procedure of cell aspiration, avoiding the nucleus. (B) PCR profile of a single ChAT-positive neuron had detectable level of all HCN channel subtypes, indicating the primer worked well. (C) Bar plot indicated the co-expression of HCN1–4 in ChAT-positive neurons detected by the multiple PCR. (D) Semi-quantitative scRT-PCR analysis of HCN channel expressed on ChIs. Note that HCN2 product was detectable with the sixth serial dilutions (2−6) of the template cellular DNA. (E) The histogram of threshold probability for ChIs expressing HCN channel subtypes. Subtypes except HCN1 showed a unimodal distribution. (F) Detection of HCN2 immunoreactivity in ChIs. Giant aspiny neuronal soma within dorsolateral striatum showed labeling of HCN2 subtype. HCN1 subtype staining was weak and individual neuron was not specifically visualized well.
The semi-quantitative RT-PCR experiment was performed in the modified two-stage amplification protocol to detect the relative abundance of HCN isoforms. Representative products amplified from serial diluted templates are shown in Figure 3D for each subtype. With Gaussian curve normalization, the HCN2 is highly translated into mRNA in ChIs. The threshold probability was best fit with a single Gaussian function (solid line) for all isoforms except HCN1. The detection threshold for HCN2 was 2−5, while it was 2−3 for HCN3 or HCN4 (Figure 3E). Considering the discrepancy between mRNA and protein expression, we further validate the protein level of HCN2 and HCN1 subtypes, which are the most and least abundant at mRNA level, through immnuohistochemical staining. As shown in Figure 3F, the use of subtype-specific antibody revealed a strong expression of HCN2 on large soma, which was identified as ChI morphologically, while HCN1 expression was relatively weak. These results confirmed that HCN2 is the main isoform expressed on ChIs.
M Receptor-Dependent Decrease of Firing Activity
To observe the effect of M receptors on the firing activity of ChIs, ACh.Cl was use to activate M receptors. It was found that brief bath-application of ACh.Cl (50 μM) caused a powerful inhibition on the spontaneous firing activity of the recorded ChIs (Figure 4A). The spiking rate was slowed down (fcontrol = 1.88 ± 0.44, fACh±Cl = 1.13 ± 0.38, Figure 4B, n = 6, p < 0.05). In the presence of ACh, the firing pattern of ChIs was altered remarkably (Figure 4C), and CV was increased significantly from 0.38 ± 0.14 of the control group to 0.54 ± 0.20 (Figure 4D, n = 6, p < 0.01). In order to exclude the nicotinic (N) receptor-mediated effect, the M receptor agonist OXO-M (10 μM) was applied. OXO-M (10 μM) displayed a similar effect as ACh.Cl (Figure 4E). The spiking pattern was changed (Figures 4E,G) and the spiking rate was slowed down (fcontrol = 1.62 ± 0.67, fOXO-M = 0.93 ± 0.35, Figure 4F, n = 11, p < 0.01). The median CV was increased from 0.41 ± 0.09 of control to 0.65 ± 0.13 in the presence of OXO-M (Figures 4G,H, n = 11, p < 0.001). Moreover, the application of OXO-M profoundly increased the duration of hyperpolarization controlled by HCN channel (Figure 4M). Application of SCO (30 μM), a specific antagonist of M receptors, did not alter the spiking frequency of ChIs (fcontrol = 1.75 ± 0.61, fSCO = 2.31 ± 0.91, Figures 4I,J, n = 9, p > 0.05). However, the inhibitory effect of OXO-M was abolished in the presence of SCO (fcontrol = 1.75 ± 0.61, fSCO+OXO−M = 2.35 ± 1.10, Figures 4I,J, n = 9, p > 0.05). The spiking pattern of ChIs was not changed (Figure 4K) and the median CV was not altered with statistical significance when both SCO and OXO-M was applied continuously (CVcontrol = 0.38 ± 0.08, CVSCO = 0.35 ± 0.05, CVSCO+OXO−M = 0.37 ± 0.09, Figure 4L, n = 9, p > 0.05). The results suggested that M receptors were involved in the negative modulation on the firing activity of ChIs.
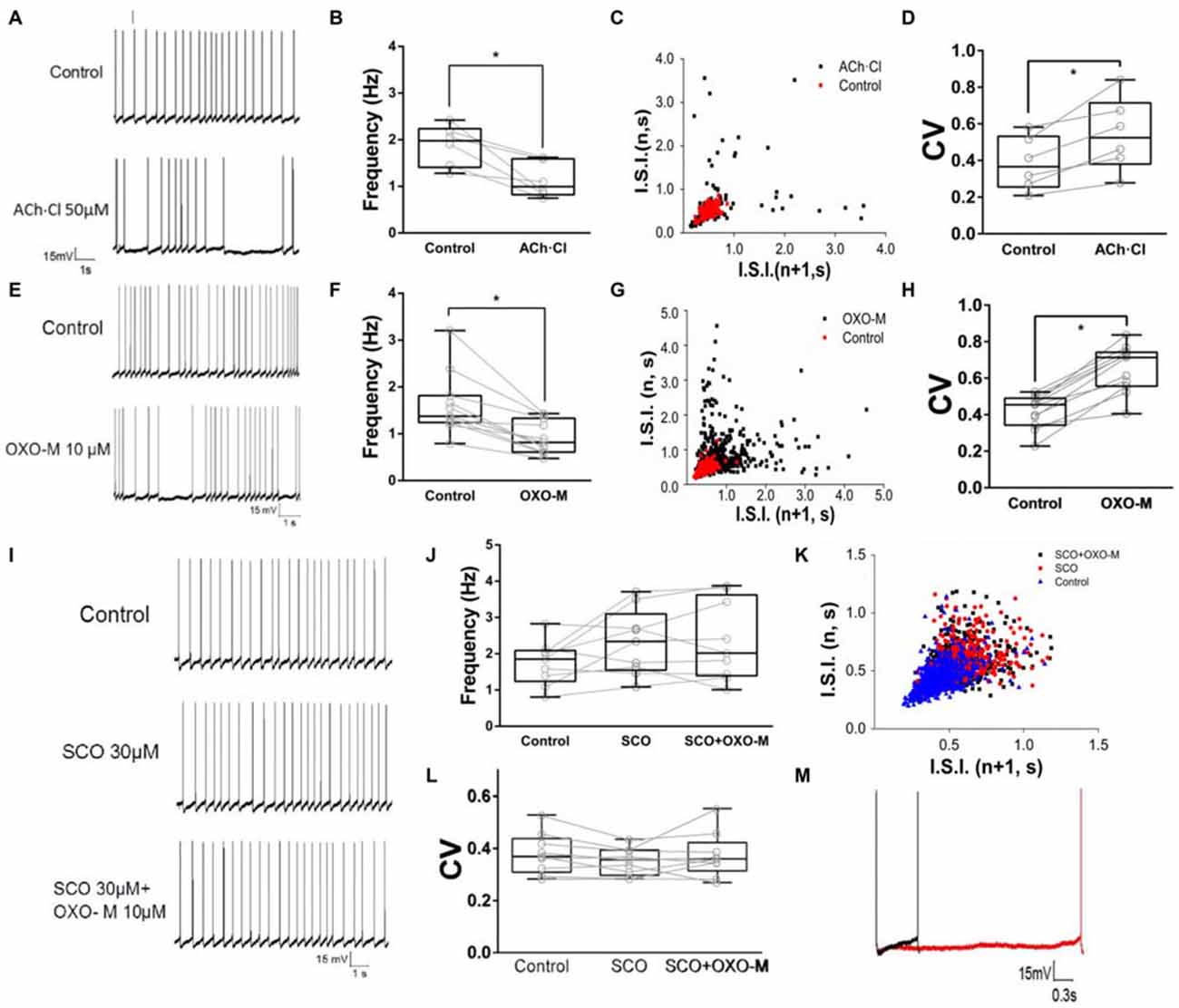
Figure 4. M receptor activation reduces firing rate of ChIs. (A) Example traces of whole-cell recordings of ChIs in control and after the application of 50 μM Acetylcholine (ACh)Cl. (B) ACh.Cl significantly decreased spontaneous firing frequency in control cells (*p < 0.05, paired student’s t-test, n = 6). (C) I.S.I. plot of the discharge of the neuron depicted in (A) (red, control; black, ACh.Cl). (D) ACh.Cl significantly increased CV of the I.S.I frequency (*p < 0.01, paired student’s t-test, n = 6). (E) Example traces of whole-cell recordings of ChIs in control and after the application of Oxotremorine (OXO-M) (10 μM). (F) OXO-M significantly decreased spontaneous firing frequency in control cells (*p < 0.01, paired student’s t-test, n = 11). (G) I.S.I. plot of the discharge of the neuron depicted in (D) (red, control; black, OXO-M). (H) OXO-M significantly increased the CV (*p < 0.001, paired student’s t-test, n = 11). (I) Example trace of whole-cell recording on ChIs in control and after the application of Scopolamine (SCO; 30 μM). SCO blocked the frequency reduction of OXO-M (10 μM). (J) SCO abolished the inhibition of OXO-M on spiking frequency while itself did not affected spontaneous firing in control cells (p > 0.05, n = 9, one-way analysis of variance (ANOVA)). (K) I.S.I. plot of the discharge of the neuron depicted in (G) (blue, control; red, SCO; black, SCO + OXO-M). (L) SCO significantly blocked the reduction effect of OXO-M on CV (p > 0.05, n = 9, one-way ANOVA analysis). (M) Amplification of the spike. Aligned action potential traces apparently showed a clear prolongation of the repolarization after the application of OXO-M (10 μM; black, control; red, OXO-M).
Muscarinic Modulation of Ihin ChIs
Previous evidence indicated that M receptor could regulate many ionic channels involved in autonomous spiking (Yan and Surmeier, 1996; Calabresi et al., 1998; Goldberg and Wilson, 2005; Ding et al., 2006). In the present study, we found that OXO-M (10 μM) inhibited Ih apparently (Figures 5A–C). A representative curve illustrated in Figure 5B indicated that the maximum inhibitory effect appeared after OXO-M was added for ~20 min. When the stimulus voltage was hyperpolarized, OXO-M exhibited significant inhibition on Ih current (Figure 5C). As shown in Figure 5D, the maximum Ih current evoked at −140 mV showed a statistical significant reduction after the addition of OXO-M. The amplitude of Ih was decreased by 21.1% ± 8.5% (Figure 5D, n = 9, p < 0.01). The peak amplitudes of tail currents were normalized and fitted by Boltzmann function to determine the voltage-dependent activation (Deng et al., 2007). As shown in Figure 5E, OXO-M caused a hyperpolarizing shift in the activation of Ih. The half-activation voltage (V1/2) was altered to −107.4 ± 2.25 mV from −102.4 ± 2.11 mV in the presence of OXO-M (n = 9, p < 0.01). SCO, 30 μM, was added into the buffer to affirm that the inhibition was mediated by the direct interaction of OXO-M on M receptors. As shown in Figures 5F,G, the time- and voltage-related inhibitory effect of OXO-M was abolished by SCO. At the maximum voltage level, OXO-M did not display statistical significant depression of Ih in the presence of SCO (99.2% ± 0.03% of control, Figure 5H, n = 5, p > 0.05). The presence of SCO occluded the alteration of V1/2 in OXO-M, while itself did not altered the V1/2 (V1/2 in control, −105.7 ± 2.23 mV; V1/2 in SCO, −104.0 ± 1.76 mV; V1/2 in SCO + OXO-M, −104.5 ± 2.50 mV; Figure 5I, n = 5, p > 0.05), which demonstrated that M receptor was involved in the Ih reduction.
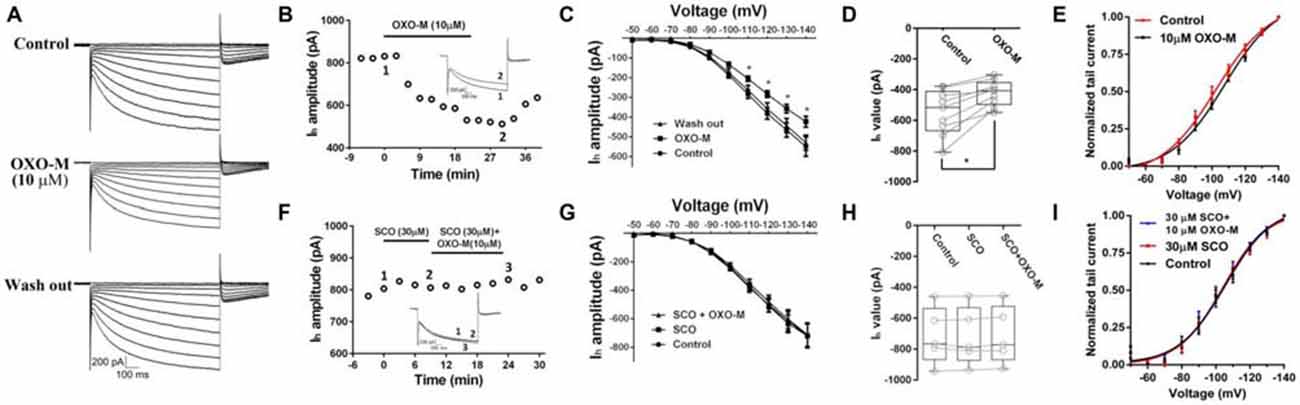
Figure 5. Inhibition of OXO-M on Ih through M receptor activation. (A) A representative trace of reversible inhibition effect of OXO-M on Ih. (B) Schematic scatter plot showed the time-related effect of OXO-M (10 μM) on Ih. (C) Ih activation I-V response curve before and after addition of OXO-M. (D) Schematic box-whiskers figure indicated the maximum current amplitude was inhibited by OXO-M at the −140 mV voltage stimulus. (E) Application of OXO-M caused a shift of voltage-dependent activation of Ih in the hyperpolarizing direction. (F) M receptor antagonist SCO (30 μM) had no discernable effect on Ih and blocked the inhibition of OXO-M (10 μM). (G) I–V response curve indicated inhibitory effect of OXO-M on Ih was blocked by M receptor antagonist. (H) Box plot summary of the change in Ih value after blocked by SCO. OXO-M can’t significantly decrease the Ih in the presence of SCO even with the maximum voltage stimulus (P > 0.05, n = 5, one-way ANOVA analysis). (I) Application of SCO blocked the shift of voltage-dependent activation of Ih, and SCO failed shift the response in the hyperpolarizing direction.
M Receptors Expression on ChIs
To determine which subtype of M receptors plays a dominant role in mediating the inhibitory effect of OXO-M, we conducted a thoroughly analysis of M receptor subtypes expressed on ChIs. Similar to the analysis of HCN subtypes, we analyzed 32 single neurons utilizing scRT-PCR technique. mRNAs of five M receptor subtypes were detected using the conventional one-stage amplification protocol (Figure 6A). The lanes of PCR products stained with ethidium bromide in 2% agarose gels indicated that M2 was the most abundant one (Figure 6A). As shown in Figure 6B, M2 receptor mRNA was expressed in all detected neurons, whereas only a small subset of neurons (20/32) had detectable levels of M4 mRNA. Meanwhile, M1-like receptors (M1, 3, 5), which were mainly expressed on projection neurons, were also detected on ChIs in a ratio of 28/32, 18/32, 26/32 respectively.
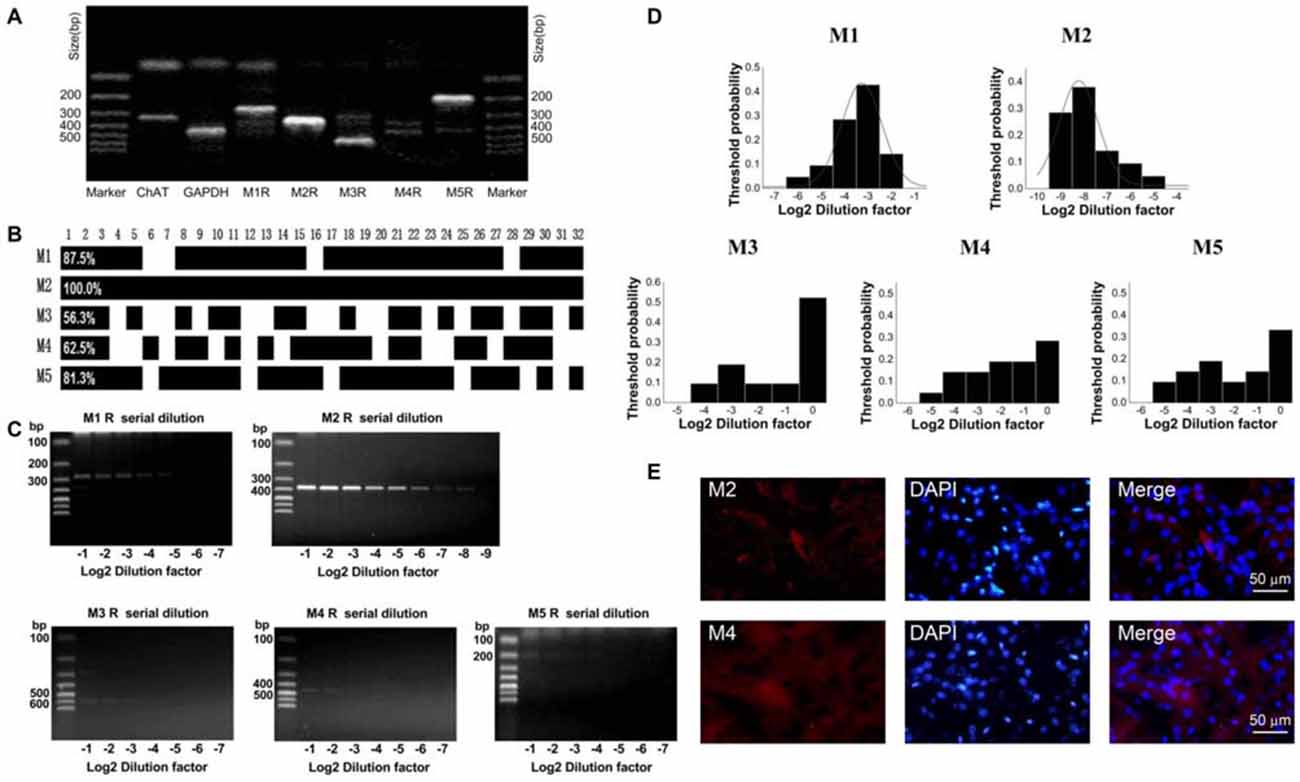
Figure 6. M2 receptor was dominantly expressed on ChIs. (A) PCR profile of a single ChAT-positive neuron that has detectable levels of all M receptor subtypes indicated the primer worked well. (B) Bar plot showed the co-expression of M1-M5 in ChAT-positive neurons. (C) Semi-quantitative scRT-PCR analysis of M receptor expressed on ChIs. Note that M2 receptor PCR amplicons were detectable with the eighth dilutions (2−8) of the template cellular DNA. (D) The histogram of threshold probability for ChIs expressing M receptor. M1 and M2 showed a unimodal distribution. (E) Giant aspiny neuronal soma within dorsolateral striatum showed strong immunoreactivity to M2 receptor, while M4 receptor immunostaining was not well visualized.
We, then performed the semi-quantitative RT-PCR experiment to discover the relative abundance of M receptors. The representative single-cell serial dilution gels were shown for each subtype in Figure 6C. M2 exhibited stronger signal than other four isoforms. The threshold probability of M1 and M2 were fit well with a single Gaussian function (solid line). As Gaussian curve normalization indicated, the detection threshold was 2−8 for M2, while 2−3 for M1, respectively (Figure 6D). The other three, M3, M4, and M5, were in low abundance that was barely above the detection level and Gaussian function could not provide an estimate of central tendency. We selected M2 and M4 to perform histochemical staining. The high expression of M2 receptor in ChIs, identified as ChI morphologically, was demonstrated by highlighted fluorescence staining. While with M4 receptor labeling, there was intense and heterogeneous staining of the striatal neuropil but individual neuron was not well visualized (Figure 6E). These results confirmed that M2 was the dominant isoform expressed on ChIs.
Gi-Protein Dependence and Interaction with M2 Receptor
To explore the mechanism underlying OXO-M inhibition on Ih, we first observed the effect of M2 receptor which is dominantly expressed on ChIs. It was found that AF-DX384, a M2-like receptor antagonist, did not show any visible effect on Ih (97.0% ± 3.8% of control, Figures 7A,G, n = 8, p > 0.05). However, the inhibitory effect of OXO-M (10 μM) on Ih was abolished after application of AF-DX384 (1 μM). The average relative Ih was changed from 78.9% ± 8.5% (Figures 7B,G, n = 9) in the OXO-M alone to 94.3% ± 5.5% in the presence of both OXO-M and AF-DX384 (Figures 7A,G, n = 6).
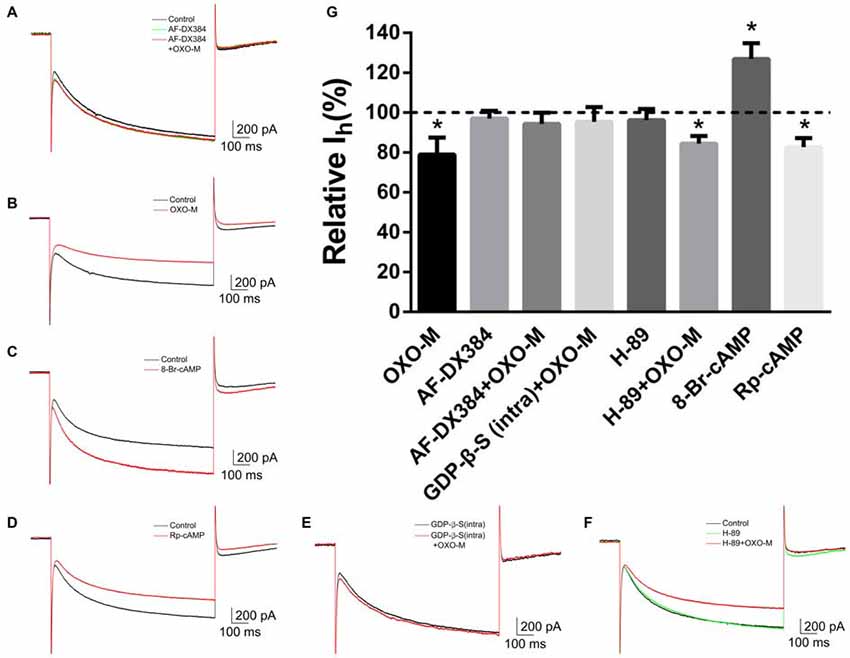
Figure 7. The inhibitory effect of OXO-M on Ih was mediated by M2 receptor activating Gi protein coupled signaling pathway. (A–F) schematic illustrations of Ih trace at the −140 mV hyperpolarizing voltage before and after application of each drug. (G) Summarized bar graph of drugs’ effect. Bath application of AF-DX384 (1 μM) did not alter Ih, but fully blocked OXO-M induced inhibition on Ih. The effect of OXO-M was fully abolished by GDP-β-S (0.5 μM), while was not affected by H-89 (10 μM). The Ih was enhanced in the presence of 8-Br-cAMP (100 μM) and reduced by Rp-cAMP (50 μM). *p < 0.01 compared with the normalized control.
To investigate the post-receptor signaling transduction, 8-Br-cAMP (100 μM), a membrane-permeable cAMP analog, was used as Deng et al. (2007) described. The amplitude of Ih current was raised to 126.8% ± 8.0% (n = 4, p < 0.01) in the presence of 8-Br-cAMP (Figures 7C,G). On the other hand, application of Rp-cAMP (50 μM), a specific inhibitor of the cAMP signaling pathway, resulted in a significant reduction of Ih (82.6% ± 4.5% of control, Figures 7D,G, n = 4, p < 0.01). GDP-β-S, an unhydrolyzable GDP analog, competes with endogenous GTP for the nucleotide binding site on G-proteins, locking G-proteins in an inactive state. When pipettes were loaded with GDP-β-S (0.5 μM), the response of Ih to OXO-M was prevented (95.4% ± 7.3% of control, Figures 7E,G, n = 7, p > 0.05). H-89 inhibits cAMP-dependent protein kinase selectively and potently. Our results revealed that H-89 (10 μM) did not present notable inhibition on Ih (96.2% ± 5.5% of control, Figures 7F,G, n = 7, p > 0.05) and co-application of H-89 (10 μM) with OXO-M (10 μM) did not hinder the inhibitory effect of OXO-M either (84.4% ± 3.8% of control, Figures 7F,G, n = 11, p < 0.01). These data support the conclusion that muscarinic inhibition of Ih is mediated through a PKA-independent cAMP pathway.
Discussion
Striatal ChIs were first identified in Kölliker (1896). Extracellular recording in the striatum of awake and normal behaving primates revealed the presence of tonically active neurons that possess particularly broad action potentials (Aosaki et al., 1995). In the past decade, studies of the ChIs have further expanded the understandings of striatal regulation and innervation in the signal input of basal ganglia circuit (Cragg, 2006; Ding et al., 2006; Pisani et al., 2007; Exley and Cragg, 2008). ChIs in the striatum have been considered a major modulator for the duration, strength, and spatial pattern of striatal MSNs output by releasing neurotransmitter ACh, which activates the postsynaptic M receptors.
As M receptors are also expressed on ChIs, we speculate that ACh, released by ChIs themselves, may affect their own excitability by activating these autoreceptors. To test this hypothesis, we identified the subtypes of M receptors expressed on ChIs and observed the effect of ACh on their firing activity. Because HCN channels are considered to contribute regulatory effects on ChIs’ excitability (Bennett et al., 2000; Wilson, 2005), we also identified the subtypes of HCN channels expressed on these neurons, and then explored the effect of M receptor agonists on Ih.
Subtypes of M Receptors and HCN Channels Expressed on ChIs
Comprising only 1–3% of all striatal neurons, ChIs have widespread and rich connections within the striatum (Woolf and Butcher, 1981; Nastuk and Graybiel, 1985). Combined morphological, histochemical, and electrophysiological features together, we confirmed the recording neurons were ChIs (Kemp and Powell, 1971; Wilson et al., 1990; Kawaguchi, 1993; Bennett et al., 2000; Wilson, 2005). We, found most of recorded neurons showed a spontaneous firing without artificial stimulation. The ChIs characteristically elicited a large amplitude and long duration AHP after repetitive firings by injected depolarizing current and displayed a pronounced hyperpolarization-activated sag potential. These features coincide with that previously reported (Calabresi et al., 1998; Bennett and Wilson, 1999; Ponterio et al., 2013).
Extensive M2 and M1 mRNAs in ChIs have been found (Bernard et al., 1992). M2-class receptors are recognized as autoreceptors, while M4 receptor is mainly expressed by a subpopulation of striatal projection neurons (Hersch et al., 1994; Bernard et al., 1999). The expression of M receptors on cultured striatal ChIs has been reported previously (Yan and Surmeier, 1996). In the present study, we analyzed the M receptor mRNA of ChIs in brain slice which is different from the report of Yan and Surmeier (1996). We, found that all five subtypes of M receptors were transcribed on ChIs. In 32 ChAT-positive neurons, the percentage of cells transcribing mRNA of M1 to M5 is 87.5%, 100.0%, 56.3%, 62.5%, and 81.3%, respectively. These results agree with the report of Yan and Surmeier (1996) that the M2-like receptors are highly transcribed while M1-like receptors are relative low in ChIs. Combining with the immunohistochemical staining results, M2 subtype is found in every ChI. Our findings are consistent with previous reports that M2 is the main subtype on ChIs (Weiner et al., 1990; Levey et al., 1991).
Earlier studies reported that ChIs expressed HCN channel subtypes of HCN2, HCN3, and HCN4 (Santoro et al., 2000; Notomi and Shigemoto, 2004), but not HCN1 (Santoro et al., 1998; Chen et al., 2001). Indeed, our data revealed that HCN2, HCN3, and HCN4 are transcribed in nearly all studied neurons by using multiplex PCR. However, HCN1 mRNA was also found on a portion of ChIs (16/27). The presence of HCN1 mRNA may due to the increments of amplified cycling number. The relative contents of all subtypes in our work are resembled with the form mentioned earlier studies. Consistent with scRT-PCR results, immunohistochemical staining directly indicated HCN2 channel fluorescence is strong and HCN1 staining is weak (Figure 4E). Among the four subtypes of HCN channels, HCN1 is activated fastest and weakly sensitive to cAMP. HCN2 has a slow activating kinetics and highest sensitivity to intracellular cAMP (Chen et al., 2001; Wang et al., 2002; Ulens and Siegelbaum, 2003). The Ih currents we recorded in ChIs display a high sensitivity to cAMP. Intracellular application of cAMP analog (8-Br-cAMP, 100 μM) enhances the current remarkably (Figure 7). These data are consistent with the notion that Ih recorded from a whole cell of striatal ChIs presents a HCN2-like characteristic.
Muscarinic Modulation of Ih in Cholinergic Interneurons
ChIs’ spiking is regulated through many ionotropic channels and G-protein coupled receptors (Yan et al., 1997; Calabresi et al., 2000; Zhou et al., 2002; Maurice et al., 2004; Wilson, 2005), and Ih plays a vital role in determining the firing rate of ChIs (Bennett and Wilson, 1999; Bennett et al., 2000; Maurice et al., 2004; Wilson, 2005). Accumulating evidence indicates that the activity of the Ih channel is regulated by a variety of neuromodulators (Pape, 1996; Frère et al., 2004). The dopaminergic modulation of Ih depends on receptor subtypes (i.e., D1- or D2- like receptors). Previous studies have shown that dopamine inhibits Ih, mediated through D2-like receptors in stratum (Deng et al., 2007) and ventral tegmental neurons (Jiang et al., 1993), whereas enhances Ih via a synergistic activation of D1- and D2- like receptors in neocortex layer I interneurons (Wu and Hablitz, 2005). Activation of β1 noradrenergic receptor or 5-HT receptor enhances Ih through a cAMP-dependent mechanism (Bobker and Williams, 1989; Pisani et al., 2003; Blomeley and Bracci, 2005; Bonsi et al., 2007; Hawkins et al., 2015). The regulation of M receptors on the spike of ChIs was also reported previously (Yan and Surmeier, 1996; Calabresi et al., 1998; Ding et al., 2006). Modulations on Na+ channel (Maurice et al., 2004), Ca2+ channel (Yan and Surmeier, 1996; Ding et al., 2006, 2010), and K+ channel (Song et al., 1998; Goldberg and Wilson, 2005) could contribute to the M receptor regulating effect. Though the interaction between M receptors and HCN channels have been hypothesized for a long time, whether Ih involved directly in the muscarinic regulation on ChIs’ spiking remained to be demonstrated.
As reported, ChIs are the main source of ACh in the striatum and produce a wide innervation over the entire striatal complex (Phelps et al., 1985; Phelps and Vaughn, 1986; Goldberg and Reynolds, 2011). Our present results demonstrate that ChIs expressed M receptor and HCN channel abundantly, and their spontaneous firing can be modulated by application of M receptor agonist or HCN channel blocker. OXO-M inhibited spiking and prolonged the AHP repolarizing time, and these correlated with a decrease in Ih current. The effect of OXO-M on Ih is blocked by application of antagonist SCO and M2-like receptor selective antagonist AF-DX384, which suggests that the activation of M2-like receptor is the prerequisite for OXO-M inhibition. Therefore, we concluded that M2-like receptors are playing a critical role in ACh-mediated effect. However, the role of which M2-like receptor, M2 or M4, plays in the ACh autoregulation needs to be demonstrated further with specific muscarinic subtype antagonists.
In our study, SCO and AF-DX384 failed to enhance Ih, while SCO slightly improved the spiking frequency of ChIs without statistical significance, as described above. These inferred that the auto-regulation mediated by presynaptic M receptor on ChIs was attenuated as previously reported (Calabresi et al., 1998). As described, the majority of ChIs are spontaneous firing in brain slices (Bennett and Wilson, 1999), there should be a background regulation of intrinsic ACh. We speculated the attenuation may induced by the following factors. Firstly, the termination of intact thalamic projection in coronal slices may attribute to the attenuation of heterosynaptic contacts and the destruction of local cholinergic circuit in striatum may result in the attenuation of hotorosynaptic contacts (Phelps et al., 1985; Hersch et al., 1994). Secondly, the whole-cell recording with low-resistance pipettes can compromise Ih likely via dilution of intracellular cAMP. In order to avoid these artificial interferences and observe the auto-inhibition directly and persuasively in electrophysiology, perhaps a cell-attached recording technique would be better suited to identifying the autoregulatory function. Otherwise, it would need in vivo patch clamp recording or/and extrinsic stimulation.
We find that both HCN channel blocker ZD7288 (Figure 2) and M receptor agonist ACh and OXO-M (Figure 4) could depress the firing activity of ChIs. However, the mechanism between the regulation of HCN channel and M receptor is unclear. The high expression of cAMP sensitive HCN channels provides a common pathway for G protein coupled receptors to regulate the ChIs activity. As previously reported, cAMP could directly combine with HCN channels on the site of CNBD domain (Pape, 1996; Wang et al., 2001; Young and Krougliak, 2004), leading to enhancement of Ih current. The reported effects of dopamine (Deng et al., 2007) and noradrenaline (Pape and McCormick, 1989; McCormick and Pape, 1990) on Ih current come to depend on the regulation of intracellular cAMP levels. These regulations are of great importance for ChIs’ spiking activity that will finally project to the output neurons of striatum (Doležal and Tuček, 1998).
As shown in Figure 7, the inhibitory effect of OXO-M is also abolished when GDP-β-S is used to fix G-proteins in an inactive state, but not while H-89 is used to inhibit cAMP-dependent protein kinase, and the effect were also mimicked in the presence of cAMP analog as reported (Deng et al., 2007). These data demonstrate that the Gi/o/cAMP signaling pathway is involved in muscarinic modulation on the excitability of ChIs, but not cAMP-dependent PKA signaling cascade. These results are consistent with the direct regulation of intracellular cAMP on HCN channel (Pedarzani and Storm, 1995; Robinson and Siegelbaum, 2003). In addition to the physiological implications for neuronal rhythmic activity, our results also suggest a novel mechanism for dynamic signaling through second messenger. It is possible that cholinergic terminals involved in this muscarinic inhibitory circuit in the striatum are associated with different behavioral contexts.
Conclusion
Our data reveal that all known subtypes of M receptors and HCN channels are transcribed in striatal ChIs. Among them, M2 and HCN2 are the most abundant ones. The spontaneous spiking of ChIs could be inhibited by extrinsic application of M receptor agonists or HCN channel blockers. Muscarinic agonists exert the inhibition on the excitability of ChIs probably by activating M2-like receptors, reducing intracellular cAMP and finally depressing HCN2 channels. These results may imply that ChIs possibly receive a negative feedback modulation by ACh in vivo.
Author Contributions
Study concept design: ZZ, JZ, LW, and XW. Collection of data: ZZ and KZ. Analysis and interpretation of data: ZZ, JZ. Drafting of the manuscript: ZZ, KZ. Critical revision of the manuscript: JZ. Study supervision: XL, HY, XM, and SZ. All authors approved the final version of the manuscript. All experiments were performed in State Key Laboratory of Toxicology and Medical Countermeasures in China.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by grants from National Integrated Drug Discovery Technology Platform Foundation of China (No.2012ZX09301003-001) and National Major Scientific and Technological Special Project for “Significant New Drug Development” (No.2014ZX09507-003). We appreciate Dr. Weifeng Yu and Dr. Haitao Wu for helpful comments and critical revision on the manuscript.
References
Alcantara, A. A., Mrzljak, L., Jakab, R. L., Levey, A. I., Hersch, S. M., and Goldman-Rakic, P. S. (2001). Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J. Comp. Neurol. 434, 445–460. doi: 10.1002/cne.1186
Aosaki, T., Kimura, M., and Graybiel, A. (1995). Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J. Neurophysiol. 73, 1234–1252.
Apicella, P., Legallet, E., and Trouche, E. (1997). Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp. Brain Res. 116, 456–466. doi: 10.1007/pl00005773
Bennett, B. D., and Bolam, J. P. (1993). Characterization of calretinin-immunoreactive structures in the striatum of the rat. Brain Res. 609, 137–148. doi: 10.1016/0006-8993(93)90866-l
Bennett, B. D., Callaway, J. C., and Wilson, C. J. (2000). Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J. Neurosci. 20, 8493–8503.
Bennett, B. D., and Wilson, C. J. (1998). Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J. Neurosci. 18, 8539–8549.
Bennett, B. D., and Wilson, C. J. (1999). Spontaneous activity of neostriatal cholinergic interneurons in vitro. J. Neurosci. 19, 5586–5596.
Bernard, V., Levey, A. I., and Bloch, B. (1999). Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation. J. Neurosci. 19, 10237–10249.
Bernard, V., Normand, E., and Bloch, B. (1992). Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J. Neurosci. 12, 3591–3600.
Biel, M., Wahl-Schott, C., Michalakis, S., and Zong, X. (2009). Hyperpolarization- activated cation channels: from genes to function. Physiol. Rev. 89, 847–885. doi: 10.1152/physrev.00029.2008
Blazquez, P. M., Fujii, N., Kojima, J., and Graybiel, A. M. (2002). A network representation of response probability in the striatum. Neuron 33, 973–982. doi: 10.1016/s0896-6273(02)00627-x
Blomeley, C., and Bracci, E. (2005). Excitatory effects of serotonin on rat striatal cholinergic interneurones. J. Physiol. 569, 715–721. doi: 10.1113/jphysiol.2005.098269
Bobker, D. H., and Williams, J. T. (1989). Serotonin augments the cationic current Ih in central neurons. Neuron 2, 1535–1540. doi: 10.1016/0896-6273(89)90041-x
Bonsi, P., Cuomo, D., Ding, J., Sciamanna, G., Ulrich, S., Tscherter, A., et al. (2007). Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6 and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32, 1840–1854. doi: 10.1038/sj.npp.1301294
Bonsi, P., Martella, G., Cuomo, D., Platania, P., Sciamanna, G., Bernardi, G., et al. (2008). Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J. Neurosci. 28, 6258–6263. doi: 10.1523/JNEUROSCI.1678-08.2008
Budde, T., Caputi, L., Kanyshkova, T., Staak, R., Abrahamczik, C., Munsch, T., et al. (2005). Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J. Neurosci. 25, 9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005
Calabresi, P., Centonze, D., Gubellini, P., Pisani, A., and Bernardi, G. (2000). Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 23, 120–126. doi: 10.1016/s0166-2236(99)01501-5
Calabresi, P., Centonze, D., Pisani, A., Sancesario, G., North, R. A., and Bernardi, G. (1998). Muscarinic IPSPs in rat striatal cholinergic interneurones. J. Physiol. 510, 421–427. doi: 10.1111/j.1469-7793.1998.421bk.x
Chamberlain, J. S., and Chamberlain, J. R. (1994). “Optimization of multiplex PCRs,” in The Polymerase Chain Reaction, eds K. B. Mullis, F. Ferré, and R. A. Gibbs (Boston: Birkhäuser), 38–46.
Chen, S., Wang, J., and Siegelbaum, S. A. (2001). Properties of hyperpolarization- activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 117, 491–504. doi: 10.1085/jgp.117.5.491
Chesselet, M., and Graybiel, A. (1986). Striatal neurons expressing somatostatin-like immunoreactivity: evidence for a peptide rgicinter neuronal system in the cat. Neuroscience 17, 547–571. doi: 10.1016/0306-4522(86)90030-8
Cimino, G. D., Metchette, K., Isaacs, S. T., and Zhu, Y. S. (1990). More false-positive problems. Nature 345, 773–774. doi: 10.1038/345773b0
Contant, C., Umbriaco, D., Garcia, S., Watkins, K., and Descarries, L. (1996). Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience 71, 937–947. doi: 10.1016/0306-4522(95)00507-2
Cowan, R. L., Wilson, C. J., Emson, P. C., and Heizmann, C. W. (1990). Parvalbumin-containing gabaergic interneurons in the rat neostriatum. J. Comp. Neurol. 302, 197–205. doi: 10.1002/cne.903020202
Cragg, S. J. (2006). Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 29, 125–131. doi: 10.1016/j.tins.2006.01.003
Deng, P., Pang, Z. P., Zhang, Y., and Xu, Z. (2005). Increase of delayed rectifier potassium currents in large aspiny neurons in the neostriatum following transient forebrain ischemia. Neuroscience 131, 135–146. doi: 10.1016/j.neuroscience.2004.11.004
Deng, P., Zhang, Y., and Xu, Z. C. (2007). Involvement of Ih in dopamine modulation of tonic firing in striatal cholinergic interneurons. J. Neurosci. 27, 3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007
Ding, J. B., Guzman, J. N., Peterson, J. D., Goldberg, J. A., and Surmeier, D. J. (2010). Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67, 294–307. doi: 10.1016/j.neuron.2010.06.017
Ding, J., Guzman, J. N., Tkatch, T., Chen, S., Goldberg, J. A., Ebert, P. J., et al. (2006). RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat. Neurosci. 9, 832–842. doi: 10.1038/nn1700
Doležal, V., and Tuček, Š. (1998). The effects of brucine and alcuronium on the inhibition of [3H] acetylcholine release from rat striatum by muscarinic receptor agonists. Br. J. Pharmacol. 124, 1213–1218. doi: 10.1038/sj.bjp.0701966
Eglen, R. M. (2012). Overview of muscarinic receptor subtypes. Handb. Exp. Pharmacol. 203, 3–28. doi: 10.1007/978-3-642-23274-9_1
Exley, R., and Cragg, S. (2008). Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br. J. Pharmacol. 153, S283–S297. doi: 10.1038/sj.bjp.0707510
Frère, S. G., Kuisle, M., and Lüthi, A. (2004). Regulation of recombinant and native hyperpolarization-activated cation channels. Mol. Neurobiol. 30, 279–305. doi: 10.1385/mn:30:3:279
Galarraga, E., Hernández-López, S., Reyes, A., Miranda, I., Bermudez-Rattoni, F., Vilchis, C., et al. (1999). Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J. Neurosci. 19, 3629–3638.
Goldberg, J. A., Ding, J. B., and Surmeier, D. J. (2012). Muscarinic modulation of striatal function and circuitry. Handb. Exp. Pharmacol. 208, 223–241. doi: 10.1007/978-3-642-23274-9_10
Goldberg, J., and Reynolds, J. (2011). Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience 198, 27–43. doi: 10.1016/j.neuroscience.2011.08.067
Goldberg, J. A., and Wilson, C. J. (2005). Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J. Neurosci. 25, 10230–10238. doi: 10.1523/JNEUROSCI.2734-05.2005
Hawkins, V. E., Hawryluk, J. M., Takakura, A. C., Tzingounis, A. V., Moreira, T. S., and Mulkey, D. K. (2015). HCN channels contribute to serotonergic modulation of ventral surface chemo sensitive neurons and respiratory activity. J. Neurophysiol. 113, 1195–1205. doi: 10.1152/jn.00487.2014
Hersch, S. M., Gutekunst, C. A., Rees, H., Heilman, C. J., and Levey, A. I. (1994). Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 14, 3351–3363.
Horikawa, K., and Armstrong, W. (1988). A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J. Neurosci. Methods 25, 1–11. doi: 10.1016/0165-0270(88)90114-8
Ince, E., Ciliax, B. J., and Levey, A. I. (1997). Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse 27, 357–366. doi: 10.1002/(sici)1098-2396(199712)27:4<357::aid-syn9>3.0.co;2-b
Jiang, Z., Pessia, M., and North, R. (1993). Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurons. J. Physiol. 462, 753–764. doi: 10.1113/jphysiol.1993.sp019580
Joshua, M., Adler, A., Mitelman, R., Vaadia, E., and Bergman, H. (2008). Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J. Neurosci. 28, 11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008
Kawaguchi, Y. (1993). Physiological, morphological and histochemical charact-erization of three classes of interneurons in rat neostriatum. J. Neurosci. 13, 4908–4923.
Kawaguchi, Y., Wilson, C. J., Augood, S. J., and Emson, P. C. (1995). Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 18, 527–535. doi: 10.1016/0166-2236(95)98374-8
Kemp, J. M., and Powell, T. (1971). The synaptic organization of the caudate nucleus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 403–412. doi: 10.1098/rstb.1971.0103
Kodirov, S. A., Wehrmeister, M., and Colom, L. V. (2014). Modulation of HCN channels in lateral septum by nicotine. Neuropharmacology 81, 274–282. doi: 10.1016/j.neuropharm.2014.02.012
Koós, T., and Tepper, J. M. (2002). Dual cholinergic control of fast-spiking interneurons in the neostriatum. J. Neurosci. 22, 529–535.
Levey, A., Kitt, C., Simonds, W., Price, D., and Brann, M. (1991). Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 11, 3218–3226.
Maurice, N., Mercer, J., Chan, C. S., Hernandez-Lopez, S., Held, J., Tkatch, T., et al. (2004). D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J. Neurosci. 24, 10289–10301. doi: 10.1523/jneurosci.2155-04.2004
McCormick, D., and Pape, H. (1990). Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurons. J. Physiol. 431, 319–342. doi: 10.1113/jphysiol.1990.sp018332
Mesulam, M., Mash, D., Hersh, L., Bothwell, M., and Geula, C. (1992). Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra and red nucleus. J. Comp. Neurol. 323, 252–268. doi: 10.1002/cne.903230209
Morris, G., Arkadir, D., Nevet, A., Vaadia, E., and Bergman, H. (2004). Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43, 133–143. doi: 10.1016/j.neuron.2004.06.012
Nastuk, M. A., and Graybiel, A. M. (1985). Patterns of muscarinic cholinergic binding in the striatum and their relation to dopamine islands and striosomes. J. Comp. Neurol. 237, 176–194. doi: 10.1002/cne.902370204
Nolan, M. F., Malleret, G., Lee, K. H., Gibbs, E., Dudman, J. T., Santoro, B., et al. (2003). The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115, 551–564. doi: 10.1016/s0092-8674(03)00884-5
Notomi, T., and Shigemoto, R. (2004). Immunohistochemical localization of Ih channel subunits, HCN1–4, in the rat brain. J. Comp. Neurol. 471, 241–276. doi: 10.1002/cne.11039
Pape, H. C. (1996). Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 58, 299–327. doi: 10.1146/annurev.physiol.58.1.299
Pape, H. C., and McCormick, D. A. (1989). Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 340, 715–718. doi: 10.1038/340715a0
Paxinos, G., and Watson, C. (1986). The Rat in Stereotaxic Coordinates. San Diego, CA: Academic Press.
Pedarzani, P., and Storm, J. F. (1995). Protein kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proc. Natl. Acad. Sci. U S A 92, 11716–11720. doi: 10.1073/pnas.92.25.11716
Phelps, P. E., Houser, C. R., and Vaughn, J. E. (1985). Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J. Comp. Neurol. 238, 286–307. doi: 10.1002/cne.902380305
Phelps, P. E., and Vaughn, J. E. (1986). Immunocytochemical localization of choline acetyltransferase in rat ventral striatum: a light and electron microscopic study. J. Neurocytol. 15, 595–617. doi: 10.1007/bf01611860
Pisani, A., Bernardi, G., Ding, J., and Surmeier, D. J. (2007). Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 30, 545–553. doi: 10.1016/j.tins.2007.07.008
Pisani, A., Bonsi, P., Centonze, D., Martorana, A., Fusco, F., Sancesario, G., et al. (2003). Activation of β1-adrenoceptors excites striatal cholinergic interneurons through a cAMP-dependent, protein kinase-independent pathway. J. Neurosci. 23, 5272–5282.
Pisani, A., Calabresi, P., Centonze, D., Marfia, G. A., and Bernardi, G. (1999). Electrophysiological recordings and calcium measurements in striatal large aspiny interneurons in response to combined O2/glucose deprivation. J. Neurophysiol. 81, 2508–2516.
Ponterio, G., Tassone, A., Sciamanna, G., Riahi, E., Vanni, V., Bonsi, P., et al. (2013). Powerful inhibitory action of mu opioid receptors (MOR) on cholinergic interneuron excitability in the dorsal striatum. Neuropharmacology 75, 78–85. doi: 10.1016/j.neuropharm.2013.07.006
Robinson, R. B., and Siegelbaum, S. A. (2003). Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65, 453–480. doi: 10.1146/annurev.physiol.65.092101.142734
Sanchez, G., Rodriguez, M. J., Pomata, P., Rela, L., and Murer, M. G. (2011). Reduction of an afterhyperpolarization current increases excitability in striatal cholinergic interneurons in rat parkinsonism. J. Neurosci. 31, 6553–6564. doi: 10.1523/JNEUROSCI.6345-10.2011
Santoro, B., Chen, S., Lüthi, A., Pavlidis, P., Shumyatsky, G. P., Tibbs, G. R., et al. (2000). Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 20, 5264–5275.
Santoro, B., Liu, D. T., Yao, H., Bartsch, D., Kandel, E. R., Siegelbaum, S. A., et al. (1998). Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93, 717–729. doi: 10.1016/s0092-8674(00)81434-8
Sciamanna, G., Tassone, A., Martella, G., Mandolesi, G., Puglisi, F., Cuomo, D., et al. (2011). Developmental profile of the aberrant dopamine D2 receptor response in striatal cholinergic interneurons in DYT1 dystonia. PLoS One 6:e24261. doi: 10.1371/journal.pone.0024261
Smith, Y., and Kieval, J. Z. (2000). Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 23, S28–S33. doi: 10.1016/s1471-1931(00)00023-9
Smith, Y., and Parent, A. (1986). Neuropeptide Y immunoreactive neurons in the striatum of cat and monkey: morphological characteristics, intrinsic organization and co-localization with somatostatin. Brain Res. 372, 241–252. doi: 10.1016/0006-8993(86)91131-5
Song, W. J., Tkatch, T., Baranauskas, G., Ichinohe, N., Kitai, S., and Surmeier, D. (1998). Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4. 2 and Kv4. 1 subunits. J. Neurosci. 18, 3124–3137.
Surmeier, D. J., Song, W. J., and Yan, Z. (1996). Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 16, 6579–6591.
Tepper, J. M., and Bolam, J. P. (2004). Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 14, 685–692. doi: 10.1016/j.conb.2004.10.003
Tkatch, T., Baranauskas, G., and Surmeier, D. J. (1998). Basal forebrain neurons adjacent to the globus pallidus co-express GABAergic and cholinergic marker mRNAs. Neuroreport 9, 1935–1939. doi: 10.1097/00001756-199806220-00004
Tkatch, T., Baranauskas, G., and Surmeier, D. J. (2000). Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J. Neurosci. 20, 579–588.
Tozzi, A., de Iure, A., Di Filippo, M., Tantucci, M., Costa, C., Borsini, F., et al. (2011). The distinct role of medium spiny neurons and cholinergic interneurons in the D2/A2A receptor interaction in the striatum: implications for Parkinson’s disease. J. Neurosci. 31, 1850–1862. doi: 10.1523/JNEUROSCI.4082-10.2011
Ulens, C., and Siegelbaum, S. A. (2003). Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry. Neuron 40, 959–970. doi: 10.1016/s0896-6273(03)00753-0
Vincent, S., Johansson, O., Hökfelt, T., Skirboll, L., Elde, R., Terenius, L., et al. (1983). NADPH-diaphorase: a selective histochemical marker for striatal neurons containing both somatostatin-and avian pancreatic polypeptide (APP) -like immunoreactivities. J. Comp. Neurol. 217, 252–263. doi: 10.1002/cne.902170303
Voorn, P., Vanderschuren, L. J., Groenewegen, H. J., Robbins, T. W., and Pennartz, C. M. (2004). Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 27, 468–474. doi: 10.1016/j.tins.2004.06.006
Wang, J., Chen, S., Nolan, M. F., and Siegelbaum, S. A. (2002). Activity-dependent regulation of HCN pacemaker channels by cyclic AMP: signaling through dynamic allosteric coupling. Neuron 36, 451–461. doi: 10.1016/s0896-6273(02)00968-6
Wang, J., Chen, S., and Siegelbaum, S. A. (2001). Regulation of hyperpolarization- activated HCN channel gating and cAMP modulation due to interactions of COOH terminus and core transmembrane regions. J. Gen. Physiol. 118, 237–250. doi: 10.1085/jgp.118.3.237
Wang, Z., Kai, L., Day, M., Ronesi, J., Yin, H. H., Ding, J., et al. (2006). Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50, 443–452. doi: 10.1016/j.neuron.2006.04.010
Weiner, D. M., Levey, A. I., and Brann, M. R. (1990). Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. U S A 87, 7050–7054. doi: 10.1073/pnas.87.18.7050
Wilson, C. J. (2005). The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron 45, 575–585. doi: 10.1016/j.neuron.2004.12.053
Wilson, C., Chang, H., and Kitai, S. (1990). Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci. 10, 508–519.
Witten, I. B., Lin, S. C., Brodsky, M., Prakash, R., Diester, I., Anikeeva, P., et al. (2010). Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681. doi: 10.1126/science.1193771
Woolf, N. J., and Butcher, L. L. (1981). Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: a combined Evans blue and acetylcholinesterase analysis. Brain Res. Bull. 7, 487–507. doi: 10.1016/0361-9230(81)90004-6
Wu, J., and Hablitz, J. J. (2005). Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J. Neurosci. 25, 6322–6328. doi: 10.1523/jneurosci.1405-05.2005
Yan, Z., Flores-Hernandez, J., and Surmeier, D. (2001). Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience 103, 1017–1024. doi: 10.1016/s0306-4522(01)00039-2
Yan, Z., and Surmeier, D. J. (1996). Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 16, 2592–2604.
Yan, Z., Song, W. J., and Surmeier, D. J. (1997). D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J. Neurophysiol. 77, 1003–1015.
Young, E. C., and Krougliak, N. (2004). Distinct structural determinants of efficacy and sensitivity in the ligand-binding domain of cyclic nucleotide-gated channels. J. Biol. Chem. 279, 3553–3562. doi: 10.1074/jbc.m310545200
Keywords: cholinergic interneurons, muscarinic receptor, HCN channel, scRT-PCR, muscarinic inhibitory
Citation: Zhao Z, Zhang K, Liu X, Yan H, Ma X, Zhang S, Zheng J, Wang L and Wei X (2016) Involvement of HCN Channel in Muscarinic Inhibitory Action on Tonic Firing of Dorsolateral Striatal Cholinergic Interneurons. Front. Cell. Neurosci. 10:71. doi: 10.3389/fncel.2016.00071
Received: 08 December 2015; Accepted: 04 March 2016;
Published: 22 March 2016.
Edited by:
Alessandro Tozzi, University of Perugia, ItalyReviewed by:
Giuseppe Sciamanna, University of Rome Tor Vergata, ItalyEnrico Bracci, University of Sheffield, UK
Manfred Josef Oswald, Central Institute for Mental Health, Germany
Copyright © 2016 Zhao, Zhang, Liu, Yan, Ma, Zhang, Zheng, Wang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyun Wang, lylywang1103@163.com;
Xiaoli Wei, weixl@bmi.ac.cn
 Zhe Zhao
Zhe Zhao Kang Zhang
Kang Zhang  Haitao Yan
Haitao Yan Xiaoyun Ma
Xiaoyun Ma Xiaoli Wei
Xiaoli Wei