Direct and indirect spino-cerebellar pathways: shared ideas but different functions in motor control
- 1Department of Integrative Medical Biology, Section of Physiology, Umeå University, Umeå, Sweden
- 2Departments of Neuroscience and Biochemistry and Molecular Biophysics, Howard Hughes Medical Institute, Kavli Institute for Brain Science, Mortimer B. Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY, USA
- 3Department of Experimental Medical Science, University of Lund, Lund, Sweden
The impressive precision of mammalian limb movements relies on internal feedback pathways that convey information about ongoing motor output to cerebellar circuits. The spino-cerebellar tracts (SCT) in the cervical, thoracic and lumbar spinal cord have long been considered canonical neural substrates for the conveyance of internal feedback signals. Here we consider the distinct features of an indirect spino-cerebellar route, via the brainstem lateral reticular nucleus (LRN), and the implications of this pre-cerebellar “detour” for the execution and evolution of limb motor control. Both direct and indirect spino-cerebellar pathways signal spinal interneuronal activity to the cerebellum during movements, but evidence suggests that direct SCT neurons are mainly modulated by rhythmic activity, whereas the LRN also receives information from systems active during postural adjustment, reaching and grasping. Thus, while direct and indirect spino-cerebellar circuits can both be regarded as internal copy pathways, it seems likely that the direct system is principally dedicated to rhythmic motor acts like locomotion, while the indirect system also provides a means of pre-cerebellar integration relevant to the execution and coordination of dexterous limb movements.
Introduction
Cerebellar circuits are of major importance in the control of movements, providing a neural basis for pattern recognition and motor behavioral correction and adaptation (Ito, 2006). These contributions to motor control depend on specific mossy fiber and climbing fiber cerebellar inputs that convey information about both ongoing motor output and external sensory events (Ito, 1984; Dean et al., 2010). In this paper, we focus on the organization of mossy fiber systems and, more specifically, we delineate two classes of spino-cerebellar pathways: direct spino-cerebellar projections, and indirect pathways via the lateral reticular nucleus (LRN; referred to as the spino-LRN-cerebellar pathway), as illustrated schematically in Figure 1.
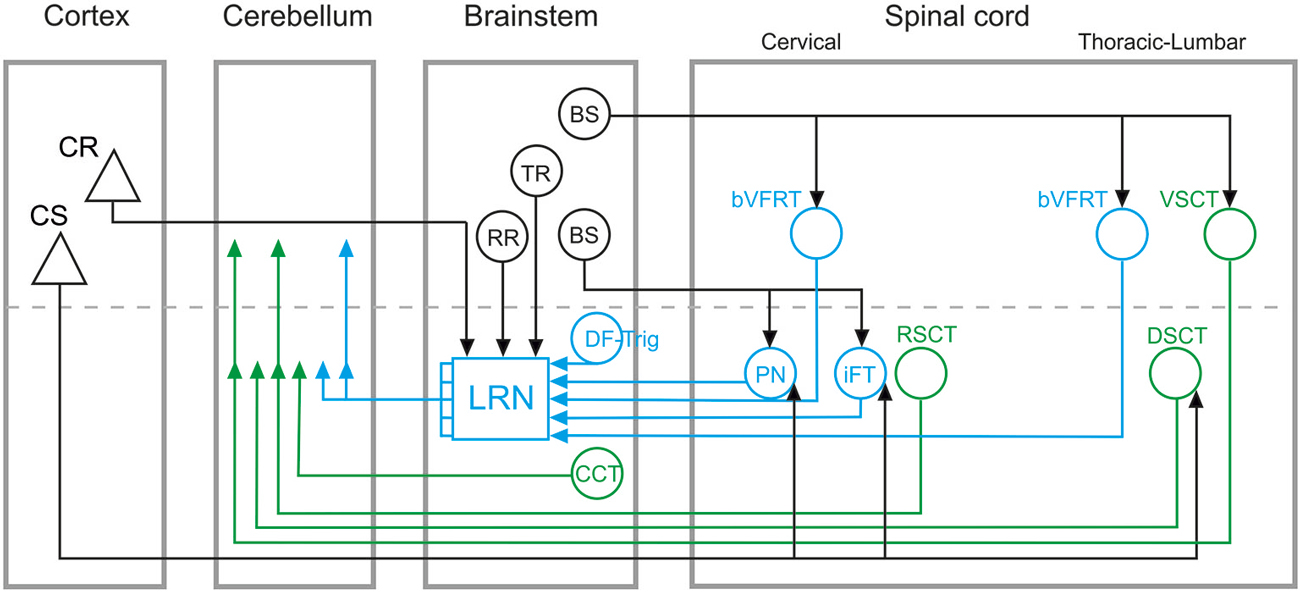
Figure 1. Overview of direct spino-cerebellar and indirect spino-LRN-cerebellar mossy fiber pathways. Direct spino-cerebellar pathways are indicated in green: the ventral spino-cerebellar tract (VSCT) and dorsal spino-cerebellar tract (DSCT) originate in thoracic and lumbar segments; the rostral spino-cerebellar tract (RSCT) originates in cervical segments; the cuneo-cerebellar tract (CCT) originates in the brainstem. Indirect spino-LRN-cerebellar pathways are indicated in blue: the bilateral ventral flexor reflex tract (bVFRT) originates in cervical and lumbar segments; the ipsilateral forelimb tract (iFT) and propriospinal neurons (PN) originate in cervical segments; and the dorsal funiculus-trigeminal tract (DF-Trig) originates in the brainstem. Green and blue arrowheads in the cerebellum indicate ipsi- and contralateral terminations (on either side of the dashed line). In the lateral reticular nucleus (LRN), discrete and convergent pathways convey information from the various spinal systems to the cerebellar cortex, though in this simplified circuit diagram they are illustrated by a combined mossy fiber output from the LRN. Descending inputs onto brainstem and spinal circuits are marked by black lines and arrowheads: cortico-spinal (CS), cortico-reticular (CR), rubro-reticular (RR), tecto-reticular (TR), and bulbo-spinal (BS). CS projections are to PN, iFT, and DSCT, but may consist of separate subpopulations. The CR, RR, and TR projections are to the LRN. The BS projections include different subpopulations: to bVFRT and VSCT mainly via the lateral vestibulo-spinal tract; to PN via the rubro-spinal, reticulo-spinal and tecto-spinal tracts; to iFT via the rubro-spinal tract.
Spino-cerebellar pathways have been implicated in the transmission of information about external events from various sensory modalities (cf. review, Stecina et al., 2013), the cancellation of reafferent sensory signals during self-generated movements (Hantman and Jessell, 2010), and the conveyance of internal copies of motor commands for rapid motor prediction and correction (Lundberg, 1971; Arshavsky et al., 1972, 1978; Alstermark and Isa, 2012; Fedirchuk et al., 2013; Azim and Alstermark, 2015). However, little is known about the organizational and functional logic underlying the existence of two separate systems for conveying spinal signals to the cerebellum. By comparing the phylogeny, anatomy, genetic identities and functional organization of direct and indirect spino-cerebellar circuits, we highlight key similarities and differences between these pathways and discuss principal questions that remain.
Phylogeny
A phylogenetic comparison of cerebellar circuits has been extensively reviewed by Ito (1984). There is evidence that direct spino-cerebellar and indirect spino-LRN-cerebellar tracts coexist in teleost fish (Szabo et al., 1990; Finger, 2000), suggesting an early evolutionary divergence of these pathways. In mammals, several direct spino-cerebellar tracts (SCT) have been identified anatomically and electrophysiologically. Two of the most studied are the dorsal (DSCT) and ventral (VSCT) spino-cerebellar tracts that originate in the thoracic and lumbar spinal cord (Jankowska et al., 2011; cf. review: Stecina et al., 2013). The corresponding direct SCT for forelimb regions are the cuneo-cerebellar tract (CCT; Jansen and Brodal, 1954; Ekerot and Larson, 1972) in the brainstem and the rostral spino-cerebellar tract (RSCT; Oscarsson, 1965; Hirai et al., 1976) in cervical segments, respectively (Figure 1).
Indirect spino-LRN-cerebellar pathways have mainly been studied in the cat (Clendenin et al., 1974a,b,c,d, 1975; Matsushita and Ikeda, 1976; Ekerot, 1990a,b,c), but comparative anatomy (Walberg, 1952) has revealed the existence of the LRN in a large number of mammals including Erinaceomorpha (hedgehog), Chiroptera (bat), Rodentia (squirrel, mouse and rat), Lagomorpha (hare), Carnivora (cat, dog and seal), Cetartiodactyla (harbor porpoise), Artiodactyla (pig, cow and roe deer) and Primates (rhesus macaque and human).
Interestingly, in teleosts there are abundant axon collaterals from direct spino-cerebellar pathways to the LRN (Szabo et al., 1990), whereas in cats the DSCT does not provide collateral excitation to LRN neurons (Ekerot and Oscarsson, 1975). These phylogenetic differences suggest that direct spino-cerebellar and indirect spino-LRN-cerebellar pathways may have originated as cooperative systems, which became progressively separated as more advanced motor repertories evolved.
Anatomy
As shown in Figure 1, direct spino-cerebellar and indirect spino-LRN-cerebellar pathways originate in cervical, thoracic and lumbar spinal segments, as well as in the brainstem (for review, cf. Alstermark and Ekerot, 2013; Pivetta et al., 2014). Within the direct and indirect classes, subpopulations with ipsilateral, contralateral and bilateral projections have been identified (cf. reviews: Alstermark and Ekerot, 2013; Stecina et al., 2013). The ultimate mossy fiber terminations of these pathways in the cerebellar cortex are found mainly in the vermal and paravermal regions of the anterior and posterior lobes, as well as in the paramedian lobe. The location of ascending axonal projections in the white matter of the spinal cord and the pattern of mossy fiber termination zones within the cerebellar cortex differ across individual systems, but broad comparison of direct and indirect pathways to each other reveals no clear differences (cf. review Ito, 1984). Thus, at least at the gross anatomical level, direct spino-cerebellar and indirect spino-LRN-cerebellar pathways target overlapping cerebellar cortical circuits.
Genetic Identities
The genetic delineation of neuronal subtypes has complemented classical anatomical and electrophysiological characterization of spinal circuits, and has provided a means for selective manipulation and functional dissection of these pathways (Goulding, 2009). While the molecular identities of each of the direct spino-cerebellar systems are yet to be fully defined, studies in mice have revealed that a population of dorsally-derived spinal interneurons that express the transcription factor Math1 give rise to multiple spino-cerebellar pathways (Bermingham et al., 2001). Moreover, DSCT neurons in Clarke’s column have been shown to selectively express the neurotrophic factor Gdnf (Hantman and Jessell, 2010).
Indirect spino-LRN-cerebellar pathways, and the cervical propriospinal neuron (PN) system in particular, have been the subject of much recent genetic scrutiny. A prominent population of excitatory PNs involved in goal-directed reaching movements was identified within the Chx10-expressing V2a interneuron class (Azim et al., 2014); notably, only cervical but not lumbar V2a interneurons project to the LRN, indicating that indirect LRN-cerebellar pathways originating in the lumbar cord have distinct genetic identities. In zebrafish, a subset of V2a spinal interneurons send ascending projections to the hindbrain (Menelaou et al., 2014), suggesting that the V2a interneuron class establishes an evolutionarily conserved circuit for the conveyance of motor signals to supraspinal regions. Moreover, recent genetic and viral labeling studies in mice have revealed that in addition to V2a interneurons, several classes of molecularly defined excitatory and inhibitory cervical spinal interneurons project to the LRN (Pivetta et al., 2014), suggesting that other indirect spino-cerebellar pathways can be dissected genetically along similar lines.
Functional Organization
It has been well documented that both direct spino-cerebellar and indirect spino-LRN-cerebellar pathways convey information related to ongoing rhythmic movements, including locomotion, scratching and respiration (cf. references in reviews by Ito, 1984; Alstermark and Ekerot, 2013; Stecina et al., 2013). Moreover, it has been proposed that the VSCT (Lundberg and Weight, 1971) and DSCT (Hantman and Jessell, 2010) monitor the excitability of spinal interneurons. Interestingly, whereas the VSCT (Fedirchuk et al., 2013) and DSCT (Stecina et al., 2013) signal mainly during the flexion phase, spino-LRN-cerebellar pathways are active throughout the entire cycle of flexion and extension (cf review by Alstermark and Ekerot, 2013), suggesting that indirect pathways convey a broader range of motor signals.
Another major difference in the functional organization of direct and indirect cerebellar pathways is that the four subsystems in the indirect spino-LRN-cerebellar pathway originating in the cervical spinal cord and brainstem (Figure 1) may be dedicated to more than just rhythmic movements (Alstermark and Ekerot, 2013). These subsystems, by monitoring the excitability of spinal interneurons, could signal information about posture (bilateral ventral flexor reflex tract; bVFRT), reaching (C3-C4 propriospinal system; PN), grasping (ipsilateral forelimb tract; iFT) and jaw opening (dorsal funiculus-trigeminal tract; DF-Trig), and their convergence in the LRN might enable the coordination of these separate motor actions into coherent and smooth movements (Alstermark and Ekerot, 2013, 2015).
Among these indirect systems, the function of C3-C4 PNs has been investigated extensively in the cat, monkey, human and recently in the mouse (Alstermark and Isa, 2012; Azim et al., 2014). These studies have shown that PNs mediate motor commands for reaching by directly modulating the activity of forelimb-innervating motor neurons, while also conveying copies of these motor commands, via axon collaterals, to the LRN. Genetic manipulation of PNs in the mouse has revealed that this internal copy pathway recruits a cerebellar-motor feedback loop, providing a plausible neural substrate for the rapid updating and correction of ongoing forelimb motor output (Azim et al., 2014; Azim and Alstermark, 2015). The current lack of selective genetic access to other spino-LRN-cerebellar pathways has precluded similar exploration of their behavioral functions, yet evidence suggests that the cervical bVFRT, iFT and PN systems provide both discrete and convergent internal feedback signals to LRN-cerebellar circuits (Alstermark and Ekerot, 2013; Pivetta et al., 2014; Huma and Maxwell, 2015), potentially enabling the coordination of forelimb and postural motor control. A companion article discusses the extensive convergence of projections from these distinct systems in the LRN, providing a pre-cerebellar center for the integration of spinal signals and their modulation by descending motor cortical pathways (Alstermark and Ekerot, 2015).
Open Questions and Future Directions
1. How do descending motor pathways modulate the direct and indirect spino-cerebellar tracts? Thus far, only the descending inputs to the cervical PN system have been investigated systematically (Alstermark and Lundberg, 1992; Alstermark and Isa, 2012; Azim et al., 2014). The convergence of descending pathways onto cervical PNs suggests a role for these neurons in integrating motor command signals and conveying copies of this information to LRN-cerebellar circuits. A better understanding of the descending inputs onto other direct and indirect cerebellar pathways should help to clarify their potential contributions to voluntary movements.
2. Which of the SCT convey internal copies of last-order interneuronal signals to motor neurons? A bifurcating pre-motor/internal copy pathway has been demonstrated for the PN system in the cat, monkey, human and mouse (Alstermark and Isa, 2012; Azim et al., 2014), and recent anatomical evidence in the mouse suggests that iFT and bVFRT systems might also send bifurcating projections directly to forelimb motor neurons and to the LRN (Pivetta et al., 2014). However, innervation of motor neurons by these pathways remains largely untested in other mammals. Studies in the cat suggest that bVFRT neurons do not project directly to lumbar motor neurons (Alstermark, Lundberg and Sybirska, unpublished findings), though direct projections to cervical motor neurons have not been explored.
3. Do any of the mammalian direct SCT send collaterals to the LRN, as they do in teleost? Studies of the DSCT suggest that collaterals to the LRN do not exist in the cat (Ekerot and Oscarsson, 1975), though additional anatomical and electrophysiological examination is needed to resolve whether the strict separation of direct and indirect cerebellar pathways is a distinguishing feature of mammalian motor circuits.
4. What is the function of the cortico-reticular (CR) projection to the LRN? This pathway may exert a modulatory top-down influence over the information conveyed from the spinal cord to the cerebellum. Genetic dissection of LRN neurons and their input pathways could help resolve the organization and function of descending control of LRN output by the cerebral cortex.
5. What are the behavioral contributions of each of the direct and indirect spino-cerebellar systems? The diversity of direct and indirect cerebellar pathways in the cervical cord in particular suggests that these systems may have evolved in concert with the increasing complexity of dexterous forelimb movements. The identification of unique genetic markers for each of these pathways should offer a means to access and manipulate these circuits selectively, providing the experimental resolution needed to characterize their discrete contributions to motor behavior (Azim et al., 2014; Azim and Alstermark, 2015).
6. There is growing interest in applying computational neurobiology approaches to understanding the molecular and genetic mechanisms that may contribute to spino-cerebellar ataxia (cf. review by Brown et al., 2015), and models devoted to the role of internal feedback more generally have explored various neural circuits in the cortex, brainstem and spinal cord (cf. review by Azim and Alstermark, 2015). Regarding spinocerebellar pathways, a hypothesis has been forwarded on their role in the multi-dimensional integration of sensorimotor information (Spanne and Jörntell, 2013). However, in this model, direct spino-cerebellar and indirect spino-LRN-cerebellar pathways are grouped together. A new hypothesis has recently been proposed that focuses specifically on the role of indirect spino-LRN-cerebellar pathways (Alstermark and Ekerot, 2013). Future modeling approaches, informed by the experimental work described above, should provide greater insight into the discrete functions of direct and indirect spino-cerebellar systems.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the Swedish Research Council and Umeå University to BA and by a National Institutes of Health (NIH) K99 award (NS088193) to EA.
References
Alstermark, B., and Ekerot, C. F. (2013). The lateral reticular nucleus: a precerebellar centre providing the cerebellum with overview and integration of motor functions at systems level. A new hypothesis. J. Physiol. 591, 5453–5458. doi: 10.1113/jphysiol.2013.256669
Alstermark, B., and Ekerot, C. (2015). The lateral reticular nucleus; integration of descending and ascending systems regulating voluntary forelimb movements. Front. Comput. Neurosci.
Alstermark, B., and Isa, T. (2012). Circuits for skilled reaching and grasping. Annu. Rev. Neurosci. 35, 559–578. doi: 10.1146/annurev-neuro-062111-150527
Alstermark, B., and Lundberg, A. (1992). “The C3-C4 Propriospinal System: Target-Reaching and Food-Taking,” in Muscle Afferents and Spinal Control of Movement, eds L. Jami, E. Pierrot-Deseilligny, and D. Zytnicki (Paris: IBRO Symposium), 327–354.
Arshavsky, Y. I., Berkinblit, M. B., Fukson, O. I., Gelfand, I. M., and Orlovsky, G. N. (1972). Origin of modulation in neurones of the ventral spinocerebellar tract during locomotion. Brain Res. 43, 276–279. doi: 10.1016/0006-8993(72)90296-x
Arshavsky, Y. I., Gelfand, I. M., Orlovsky, G. N., and Pavlova, G. A. (1978). Messages conveyed by spinocerebellar pathways during scratching in the cat. I. Activity of neurons of the lateral reticular nucleus. Brain Res. 151, 479–491. doi: 10.1016/0006-8993(78)91081-8
Azim, E., and Alstermark, B. (2015). Skilled forelimb movements and internal copy motor circuits. Curr. Opin. Neurobiol. 9, 16–24. doi: 10.1016/j.conb.2014.12.009
Azim, E., Jiang, J., Alstermark, B., and Jessell, T. M. (2014). Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 508, 357–363. doi: 10.1038/nature13021
Bermingham, N. A., Hassan, B. A., Wang, V. Y., Fernandez, M., Banfi, S., Bellen, H. J., et al. (2001). Proprioceptor pathway development is dependent on MATH1. Neuron. 30, 411–422. doi: 10.1016/s0896-6273(01)00305-1
Brown, S. A., McCullough, L. D., and Loew, L. M. (2015). Computational neurobiology is a useful tool in translational neurology: the example of ataxia. Front. Neurosci. 9:1. doi: 10.3389/fnins.2015.00001
Clendenin, M., Ekerot, C. F., and Oscarsson, O. (1974d). The lateral reticular nucleus in the cat. III. Organization of component activated from ipsilateral forelimb tract. Exp. Brain Res. 21, 501–513. doi: 10.1007/bf00237168
Clendenin, M., Ekerot, C. F., and Oscarsson, O. (1975). The lateral reticular nucleus in the cat. IV. Activation from dorsal funiculus and trigeminal afferents. Exp. Brain Res. 24, 131–144. doi: 10.1007/bf00234059
Clendenin, M., Ekerot, C. F., Oscarsson, O., and Rosén, I. (1974a). Functional organization of two spinocerebellar paths relayed through the lateral reticular nucleus in the cat. Brain Res. 69, 140–143. doi: 10.1016/0006-8993(74)90379-5
Clendenin, M., Ekerot, C. F., Oscarsson, O., and Rosén, I. (1974b). The lateral reticular nucleus in the cat. I. Mossy fibre distribution in cerebellar cortex. Exp. Brain Res. 21, 473–486. doi: 10.1007/bf00237166
Clendenin, M., Ekerot, C. F., Oscarsson, O., and Rosén, I. (1974c). The lateral reticular nucleus in the cat. II. Organization of component activated from bilateral ventral flexor reflex tract (bVFRT). Exp. Brain Res. 21, 487–500. doi: 10.1007/bf00237167
Dean, P., Porrill, J., Ekerot, C. F., and Jörntell, H. (2010). The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat. Rev. Neurosci. 11, 30–43. doi: 10.1038/nrn2756
Ekerot, C. F. (1990a). The lateral reticular nucleus in the cat. VI. Excitatory and inhibitory afferent paths. Exp. Brain Res. 79, 109–119. doi: 10.1007/bf00228879
Ekerot, C. F. (1990b). The lateral reticular nucleus in the cat. VII. Excitatory and inhibitory projection from the ipsilateral forelimb tract (iF tract). Exp. Brain Res. 79, 120–128. doi: 10.1007/BF00228880
Ekerot, C. F. (1990c). The lateral reticular nucleus in the cat. VIII. Excitatory and inhibitory projection from the bilateral ventral flexor reflex tract (bVFRT). Exp. Brain Res. 79, 129–137. doi: 10.1007/BF00228881
Ekerot, C. F., and Larson, B. (1972). Differential termination of the exteroceptive and proprioceptive components of the cuneocerebellar tract. Brain Res. 36, 420–424. doi: 10.1016/0006-8993(72)90748-2
Ekerot, C. F., and Oscarsson, O. (1975). Inhibitory spinal paths to the lateral reticular nucleus. Brain Res. 99, 157–161. doi: 10.1016/0006-8993(75)90619-8
Fedirchuk, B., Stecina, K., Kristensen, K. K., Zhang, M., Meehan, C. F., Bennett, D. J., et al. (2013). Rhythmic activity of feline dorsal and ventral spinocerebellar tract neurons during fictive motor actions. J. Neurophysiol. 109, 375–388. doi: 10.1152/jn.00649.2012
Finger, T. E. (2000). Ascending spinal systems in the fish, Prionotus carolinus. J. Comp. Neurol. 422, 106–122. doi: 10.1002/(SICI)1096-9861(20000619)422:1%3C106::AID-CNE7%3E3.0.CO;2-T
Goulding, M. (2009). Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518. doi: 10.1038/nrn2608
Hantman, A. W., and Jessell, T. M. (2010). Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat. Neurosci. 13, 1233–1239. doi: 10.1038/nn.2637
Hirai, N., Hongo, T., Kudo, N., and Yamaguchi, T. (1976). Heterogeneous composition of the spinocerebellar tract originating from the cervical enlargement in the cat. Brain Res. 109, 387–391. doi: 10.1016/0006-8993(76)90539-4
Huma, Z., and Maxwell, D. J. (2015). The spino-bulbar-cerebellar pathway: organization and neurochemical properties of spinal cells that project to the lateral reticular nucleus in the rat. Front. Neuroanat. 9:1. doi: 10.3389/fnana.2015.00001
Ito, M. (2006). Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 78, 272–303. doi: 10.1016/j.pneurobio.2006.02.006
Jankowska, E., Nilsson, E., and Hammar, I. (2011). Processing information related to centrally initiated locomotor and voluntary movements by feline spinocerebellar neurones. J. Physiol. 589, 5709–5725. doi: 10.1113/jphysiol.2011.213678
Lundberg, A. (1971). Function of the ventral spinocerebellar tract. A new hypothesis. Exp. Brain Res. 12, 317–330. doi: 10.1007/bf00237923
Lundberg, A., and Weight, F. (1971). Functional organization of connexions to the ventral spinocerebellar tract. Exp. Brain Res. 12, 295–316. doi: 10.1007/bf00237922
Matsushita, M., and Ikeda, M. (1976). Projections from the lateral reticular nucleus to the cerebellar cortex and nuclei in the cat. Exp. Brain Res. 24, 403–421. doi: 10.1007/bf00235006
Menelaou, E., VanDunk, C., and McLean, D. L. (2014). Differences in the morphology of spinal V2a neurons reflect their recruitment order during swimming in larval zebrafish. J. Comp. Neurol. 522, 1232–1248. doi: 10.1002/cne.23465
Oscarsson, O. (1965). Functional Organization of the Spino- and Cuneocerebellar Tracts. Physiol. Rev. 45, 495–522.
Pivetta, C., Esposito, M. S., Sigrist, M., and Arber, S. (2014). Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell. 156, 537–548. doi: 10.1016/j.cell.2013.12.014
Spanne, A., and Jörntell, H. (2013). Processing of multi-dimensional sensorimotor information in the spinal and cerebellar neuronal circuitry: a new hypothesis. PloS Comput. Biol. 9:e1002979. doi: 10.1371/journal.pcbi.1002979
Stecina, K., Fedirchuk, B., and Hultborn, H. (2013). Information to cerebellum on spinal motor networks mediated by the dorsal spinocerebellar tract. J. Physiol. 591, 5433–5443. doi: 10.1113/jphysiol.2012.249110
Szabo, T., Libouban, S., and Denizot, J. P. (1990). A well defined spinocerebellar system in the weakly electric teleost fish Gnathonemus petersii. A tracing and immuno-histochemical study. Arch. Ital. Biol. 128, 229–247.
Keywords: lateral reticular nucleus (LRN), spino-cerebellar pathways, spino-LRN-cerebellar pathways, internal feedback, motor control
Citation: Jiang J, Azim E, Ekerot C-F and Alstermark B (2015) Direct and indirect spino-cerebellar pathways: shared ideas but different functions in motor control. Front. Comput. Neurosci. 9:75. doi: 10.3389/fncom.2015.00075
Received: 13 March 2015; Accepted: 01 June 2015;
Published: 06 July 2015.
Edited by:
Ning Lan, Shanghai Jiao Tong University, ChinaReviewed by:
Irina N. Beloozerova, Barrow Neurological Institute, USAVincent C. K. Cheung, The Chinese University of Hong Kong, Hong Kong
Copyright © 2015 Jiang, Azim, Ekerot and Alstermark. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bror Alstermark, Department of Integrative Medical Biology, Section of Physiology, Umeå University, Linneus väg 9, 901 87 Umeå, Sweden, bror.alstermark@umu.se
† These authors have contributed equally to this work.
 Juan Jiang
Juan Jiang Eiman Azim
Eiman Azim Carl-Fredrik Ekerot3†
Carl-Fredrik Ekerot3†  Bror Alstermark
Bror Alstermark