Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2014; 23(2): 155-162
Published online June 30, 2014
https://doi.org/10.5607/en.2014.23.2.155
© The Korean Society for Brain and Neural Sciences
Lipocalin-2 Acts as a Neuroinflammatogen in Lipopolysaccharide-injected Mice
Myungwon Jin1,2, Eunha Jang1,2 and Kyoungho Suk1,2*
1Department of Pharmacology, Brain Science & Engineering Institute, 2Department of Biomedical Sciences, BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University School of Medicine, Daegu 700-422, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-53-420-4835, FAX: 82-53-256-1566
e-mail: ksuk@knu.ac.kr
Lipocalin-2 (LCN2) is a key mediator of various cellular processes. Recent studies have indicated that LCN2 also plays an important role in central nervous system (CNS) injuries and neurological diseases, such as spinal cord injury, stroke, experimental autoimmune encephalomyelitis, and neurodegenerative diseases. Here, we investigated the role of LCN2 in a rodent model of lipopolysaccharide (LPS)-induced neuroinflammation. At 24 hours after intraperitoneal injection of LPS, LCN2 expression was strongly induced in the brain; LCN2 was mainly expressed in endothelial cells, astrocytes, and microglia. Next, we used LCN2-deficient mice to further investigate the role of LCN2 in neuroinflammation. LCN2 deficiency attenuated LPS-induced glial activation in the brain. In a mechanistic study employing glia/neuron co-cultures, LCN2 deficiency reduced glial neurotoxicity. Our results indicate that LCN2 plays a central role in the neuroinflammatory responses following LPS administration, and that LCN2 might contribute to the uncontrolled neurotoxic glial activation under excessive and chronic inflammatory conditions.
Keywords: astrocyte, lipocalin-2, neuron, neuroinflammation, neuroinflammatogen
Inflammation is a key component of various acute and chronic central nervous system (CNS) disorders [1]. There is much evidence that inflammation and inflammatory mediators contribute to the pathogenesis of CNS injuries and neurological diseases [2], including stroke [3], traumatic brain injury [4], Alzheimer's disease [5], Parkinson's disease [6, 7], and multiple sclerosis [8]. Lipocalin-2 (LCN2) is a 25 kDa glycoprotein and a member of the lipocalin family [9, 10]. It plays a regulatory role in diverse cellular processes, such as cell death/survival [11], cell migration/invasion [12], cell differentiation [13], and iron delivery [14, 15]. Nevertheless, the role of LCN2 in the CNS is not well understood. We have recently reported that glial cells in the CNS express LCN2. LCN2 regulates glial cell death/survival, motility, morphology [16, 17], and functional polarization [18, 19]. It also has a critical role as a chemokine inducer [20] and a mediator of neuronal cell death in the CNS [21, 22]. LCN2 has been proposed to be linked to experimental autoimmune encephalomyelitis [23], intracerebral hemorrhage [24], spinal cord injury [25], chronic inflammatory pain [26], neuropathic pain [27], and ischemic stroke [28].
In the present study, we investigated the role of LCN2 in neuroinflammation by comparing inflammation-associated events in wild-type (WT) and LCN2-deficient mice after lipopolysaccharide (LPS) injection. Additional mechanistic studies were conducted using glia/neuron co-cultures. Our results suggest that LCN2 is a neuroinflammatogen that contributes to the initiation and maintenance of CNS inflammation by activating glia cells and promoting neurotoxicity.
MATERIALS AND METHODS
LCN2-knockout (KO) mice were provided by Dr. Kiyoshi Mori (Kyoto University, Japan) and Dr. Shizuo Akira (Osaka University, Japan). The genetic background of the LCN2-KO mice was C57BL/6 [14]. WT and LCN2-KO mice were backcrossed for 8-10 generations onto a C57BL/6 background to generate homozygous and heterozygous animals free of background phenotypic effects. The absence of LCN2 was confirmed by PCR analysis of genomic DNA. All animal procedures were approved by the Institutional Animal Care Committee of Kyungpook National University and performed in accordance with the animal care guidelines of the National Institute of Health. All efforts were made to minimize the number of animals used as well as animal suffering. Mice were housed under specific pathogen-free conditions within the Kyungpook National University School of Medicine.
A peripheral injection of LPS was administered to evoke neuroinflammation in mice as previously described [29]. Mice were administered an intraperitoneal (i.p.) injection of vehicle or LPS (5 mg/kg) (Sigma-Aldrich, St Louis, MO, USA). Animals in the vehicle control group were administered the same volume of saline. Animals were put under deep ether-induced anesthesia 24 h after the i.p. injection and then killed.
Mice were deeply anesthetized with an ether overdose and perfused through the aorta with DEPC-treated PBS in order to remove blood. Brains were rapidly excised and divided into two hemispheres, which were individually homogenized in Trizol (Invitrogen, Carlsbad, CA, USA). Total RNA (2 µg) from each sample was reverse transcribed into cDNA using a First Strand cDNA Synthesis Kit (MBI Fermentas, Hanover, Germany). Real-time reverse transcription-PCR (RT-PCR) was performed using the One-step SYBR® PrimeScript™ RT-PCR kit (Perfect Real Time; Takara Bio, Tokyo) and the ABI Prism® 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. The 2-ΔΔCT method was used to calculate relative changes in gene expression determined by real-time RT-PCR. GAPDH was used as a reference gene. Traditional PCR amplification using specific primer sets was carried out at an annealing temperature of 55~60℃ for 25~32 cycles. PCR was performed using a C1000 Touch™ Thermal Cycler (Bio-Rad, Hercules, CA, USA). For PCR product analysis, 10 µL of the reaction was separated in agarose gel and detected under ultraviolet light following ethidium bromide staining. The nucleotide sequences of the primers used were based on published cDNA sequences (Table 1).
Mice were killed by ether inhalation, and intracardiac perfusion-fixation was performed using 0.9% NaCl and 4% paraformaldehyde (PFA) dissolved in 100 mmol/L PBS (pH 7.4). Isolated brains were immersion-fixed in 4% PFA for 72 h and cryoprotected by incubation in 30% sucrose diluted in 0.1 M PBS for 72 hr. The brains were then embedded in OCT compound (Tissue-Tek, Torrance, CA, USA). Frozen brains were cut into 20-µm-thick coronal sections, which were permeabilized in 0.1% Triton X-100 and blocked with 1% BSA and 5% normal donkey serum for 60 min at room temperature. Brain sections were then incubated with primary antibodies (goat anti-LCN2 [1:500], mouse anti-NeuN [1:200; Millipore, Billerica, MA, USA], rabbit anti-GFAP [1:500; DakoCytomation, Glostrup, Denmark], rat anti-CD31 [1:200; BD, Franklin Lakes, NJ, USA], and rabbit anti-Iba-1 [1:500; Wako, Tokyo, Japan]) overnight at 4℃. The next day, sections were washed in PBS containing 0.1% Tween 20 and then incubated with FITC- or Cy3-conjugated secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA, USA). Sections were then mounted and counterstained using gelatin containing DAPI. Tiled images of each section were captured with a CCD color video camera (Olympus D70) through a 200×objective lens attached to a fluorescence microscope (Olympus BX51). Three squares (500 µm×500 µm) were placed in the subthreshold images of six independent coronal sections. Cells in these three squares were counted and statistically analyzed using the NIH Image J program as previously described [30].
Neonatal astrocyte cultures were prepared from mixed glial cultures, as previously described [17]. Primary cultures of dissociated cerebral cortical neurons were prepared from embryonic day 15 (E15) mice, as described previously [22, 31].
For astrocyte/neuron co-cultures, cortical neurons were plated at a density of 2×105 cells per well in 24-well companion plates, and allowed to settle at 37℃ in a 5% CO2 atmosphere for 7 days. Primary astrocytes were separately plated at 2×105 cells per well in cell culture inserts (0.4-µm pore size; BD) and allowed to settle at 37℃ in a 5% CO2 atmosphere overnight. Astrocytes were pretreated with LPS (100 ng/ml) plus IFN-γ (50 U/ml) for 24 h and subsequently washed. Cell culture inserts containing astrocytes were then transferred into wells containing cortical neurons. After a 24~72-h incubation, the viability of neurons was measured.
Cortical neurons were co-cultured with astrocytes. After the indicated times, culture inserts containing astrocytes were removed and neuronal viability was measured using a MTT assay. In brief, after culture media were removed, MTT (0.5 mg/ml; Sigma-Aldrich) was added, and neurons were incubated at 37℃ in a 5% CO2 incubator. Insoluble formazan crystals were completely dissolved in DMSO, and absorbance at 570 nm was measured using a microplate reader (Anthos Labtec Instruments, Wals, Austria).
Data are presented as the mean±standard deviation (SD) of three or more independent experiments, unless otherwise stated. Two-group comparisons were analyzed using a two-tailed Student's
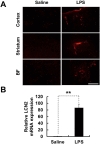
We examined the protein expression of LCN2 in the brain following an i.p. injection of LPS. A significant LCN2 expression was not detected in the brain of saline-injected mice (Fig. 1A;
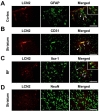
To determine the cellular distribution of LCN2 in the brain after LPS injection, we performed double-immunostaining for LCN2 and different cell-type specific markers at 24 h after LPS injection. Glial fibrillary acidic protein (GFAP), CD31, and ionized calcium-binding adapter molecule (Iba-1) were used as markers for astrocytes, endothelial cells, and microglia, respectively. LCN2 expression was colocalized with astrocytes and endothelial cells within the cortex and striatum (Fig. 2A, B). On the other hand, LCN2 expression was colocalized with microglia within basal forebrain (Fig. 2C). LCN2 was not colocalized with neurons (Fig. 2D).
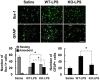
Glial activation is one of the main events in neuroinflammation. To determine the role of LCN2 during the inflammatory response seen in the brain after LPS stimulation, we compared glial activation by immunofluorescence staining in LCN2-KO mice and WT animals. LCN2 deficiency reduced the number of amoeboid microglia (representing activated microglia) after LPS injection. The number of activated astrocytes was also significantly lower in LCN2-KO mice compared to WT mice (Fig. 3).
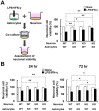
In previous studies, astrocytes were identified as one of the major cell types expressing LCN2 [16, 17, 26]. It is also known that LCN2 contributes to neuronal cell death [21]. Thus, to determine whether LCN2 directly mediates neuronal cell death, we performed astrocyte/neuron co-cultures after LPS/IFN-γ stimulation. Absence of LCN2 reduced the neurotoxicity of LPS/IFN-γ-stimulated astrocytes in co-culture at the 48-hr time point. This observation suggests that glia-derived LCN2 promotes neurotoxicity under inflammatory conditions (Fig. 4A). In addition, the absence of LCN2 in neurons also reduced neuronal cell death. Similar results were obtained after co-culturing LPS/IFN-γ-stimulated astrocytes and neurons for 24 or 72 h (Fig. 4B). These results indicate that LCN2 plays a critical role in glial neurotoxicity under prolonged inflammatory conditions.
In the present study, we examined the role of LCN2 in the brain after systemic LPS administration. After an i.p. LPS injection, the LCN2 protein was highly expressed in several brain regions. This upregulation was further confirmed at mRNA level from whole brain lysates. The LCN2 protein was detected in endothelial cells, astrocytes, and microglia within various brain regions after LPS injection. LCN2 deficiency attenuated the activation of astrocytes and microglia after LPS injection. In astrocyte/neuron co-cultures, LCN2 deficiency reduced neuronal cell death. These results indicate that LCN2 can be considered as a neuroinflammatogen.
LCN2 participates in the pathogenesis of chronic kidney disease [33], cancer [34], obesity [35], cardiovascular disease [36], and acute endotoxemia [36]. However, the role of LCN2 in the CNS is poorly defined. Recent studies indicate that LCN2 is closely associated with CNS injuries, such as chronic inflammatory pain [26], spinal cord injury [25], and ischemic stroke [28]. Here, we used a systemic inflammation model to investigate the role of LCN2 in brain inflammation. Using this model, we observed a prominent induction of LCN2 expression after LPS injection, a marked reduction of glial activation in LCN2-KO mice, and an abolishment of glial neurotoxicity in LCN2-KO mice. Together, these data indicate that LCN2 mediates neuroinflammatory responses and contributes to harmful neurotoxicity. In the previous study, LCN2 mediated apoptosis through its receptor 24p3R [15], and 24p3R was also detected on the surface of neurons [21, 22]. Thus, we speculate that LCN2 may exert neurotoxic effects through 24p3R in neuroinflammation. Our results are consistent with previous findings showing that the recombinant LCN2 protein was toxic to neurons in culture [22] and the astrocytic LCN2 protein promoted neuronal death [21]. LCN2 deficiency in neurons reduced neuronal cell death. It has been reported that neurons are also the cellular source of LCN2 in the CNS [27, 37]. Thus, neuron-derived LCN2 may have neurotoxic effects in an autocrine manner. This is, however, the subject of further investigation in our laboratory.
In summary, using a LPS-induced inflammation model, we suggest that LCN2 may act as a neuroinflammatogen in the inflamed brain. Here, LCN2 might be responsible for the amplification of an undesirable glial activation with the unintended consequence of neuronal death. LCN2 can, therefore, be considered a novel therapeutic target for future treatment of various CNS injuries and neurological disorders.
- Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci 2003;358:1669-1677.
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006;147:S232-S240.
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol 2007;184:53-68.
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock 2001;16:165-177.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging 2000;21:383-421.
- Tufekci KU, Meuwissen R, Genc S, Genc K. Inflammation in Parkinson's disease. Adv Protein Chem Struct Biol 2012;88:69-132.
- Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord 2012;18:S210-S212.
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009;132:1175-1189.
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 2002;10:1033-1043.
- Flower DR. The lipocalin protein family: structure and function. Biochem J 1996;318:1-14.
- Kehrer JP. Lipocalin-2: pro- or anti-apoptotic?. Cell Biol Toxicol 2010;26:83-89.
- Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A 2009;106:3913-3918.
- Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis 2008;52:595-605.
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004;432:917-921.
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 2005;123:1293-1305.
- Ip JP, Noçon AL, Hofer MJ, Lim SL, Muller M, Campbell IL. Lipocalin 2 in the central nervous system host response to systemic lipopolysaccharide administration. J Neuroinflammation 2011;8:124.
- Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci 2009;29:234-249.
- Jang E, Kim JH, Lee S, Kim JH, Seo JW, Jin M, Lee MG, Jang IS, Lee WH, Suk K. Phenotypic polarization of activated astrocytes: the critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. J Immunol 2013;191:5204-5219.
- Jang E, Lee S, Kim JH, Kim JH, Seo JW, Lee WH, Mori K, Nakao K, Suk K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J 2013;27:1176-1190.
- Lee S, Kim JH, Kim JH, Seo JW, Han HS, Lee WH, Mori K, Nakao K, Barasch J, Suk K. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem 2011;286:43855-43870.
- Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A 2013;110:4069-4074.
- Lee S, Lee WH, Lee MS, Mori K, Suk K. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology. J Neurosci Res 2012;90:540-550.
- Berard JL, Zarruk JG, Arbour N, Prat A, Yong VW, Jacques FH, Akira S, David S. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia 2012;60:1145-1159.
- Dong M, Xi G, Keep RF, Hua Y. Role of iron in brain lipocalin 2 upregulation after intracerebral hemorrhage in rats. Brain Res 2013;1505:86-92.
- Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, Akira S, David S. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J Neurosci 2011;31:13412-13419.
- Jha MK, Jeon S, Jin M, Ock J, Kim JH, Lee WH, Suk K. The pivotal role played by lipocalin-2 in chronic inflammatory pain. Exp Neurol 2014;254:41-53.
- Jeon S, Jha MK, Ock J, Seo J, Jin M, Cho H, Lee WH, Suk K. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury. J Biol Chem 2013;288:24116-24127.
- Jin M, Kim JH, Jang E, Lee YM, Han HS, Woo DK, Park DH, Kook H, Suk K. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 2014
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007;55:453-462.
- Lee S, Jang E, Kim JH, Lee WH, Suk K. Lipocalin-type prostaglandin D2 synthase protein regulates glial cell migration and morphology through myristoylated alanine-rich C-kinase substrate: prostaglandin D2-independent effects. J Biol Chem 2012;287:9414-9428.
- Ock J, Han HS, Hong SH, Lee SY, Han YM, Kwon BM, Suk K. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br J Pharmacol 2010;159:1646-1662.
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149-1160.
- Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 2010;120:4065-4076.
- Yang J, Moses MA. Lipocalin 2: a multifaceted modulator of human cancer. Cell Cycle 2009;8:2347-2352.
- Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 2010;59:872-882.
- Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin Chem Lab Med 2012;50:1533-1545.
- Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc Natl Acad Sci U S A 2011;108:18436-18441.